Key Insights
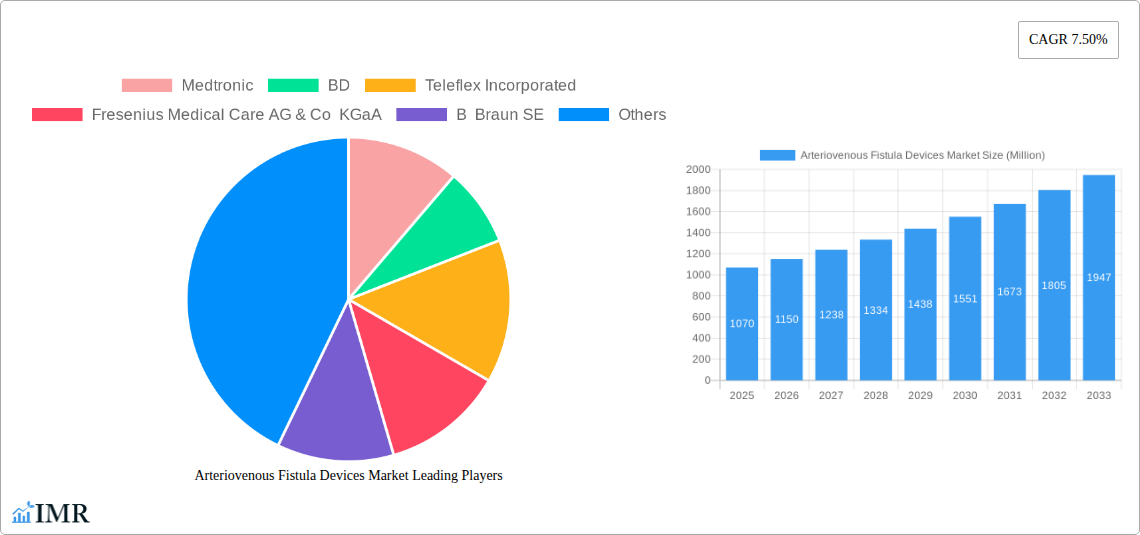
The Arteriovenous Fistula (AVF) Devices market, valued at $1.07 billion in 2025, is projected to experience robust growth, driven by the increasing prevalence of chronic kidney disease (CKD) and end-stage renal disease (ESRD) globally. The rising geriatric population, a major risk factor for CKD, significantly fuels market demand. Technological advancements in AVF creation and management, including minimally invasive techniques and improved cannulation devices, are contributing to market expansion. Furthermore, the growing preference for home hemodialysis, which often relies on well-functioning AVFs, is bolstering market growth. However, challenges remain, including the high failure rate of AVFs due to thrombosis and stenosis, which necessitates interventions and potentially impacts market growth. The market is segmented by device type (e.g., needles, catheters, grafts), material (e.g., PTFE, polyurethane), and end-user (hospitals, dialysis centers). Competition is fierce among established players like Medtronic, BD, and Fresenius Medical Care, who are continuously innovating to improve device performance and patient outcomes. The forecast period (2025-2033) suggests sustained growth, primarily driven by improved healthcare infrastructure and increased awareness of CKD management in developing economies.
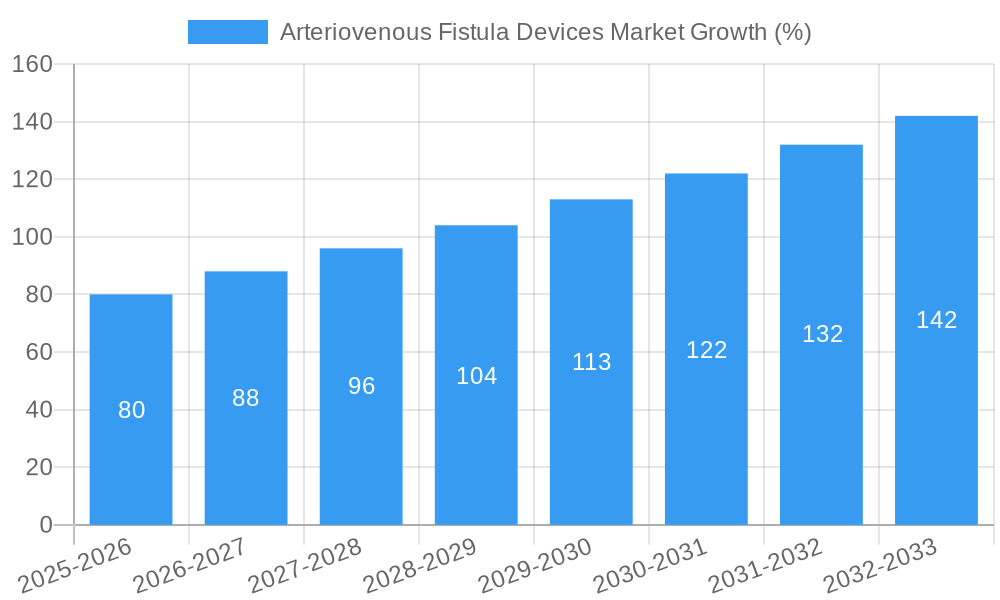
The projected Compound Annual Growth Rate (CAGR) of 7.5% from 2025 to 2033 indicates a substantial market expansion. This growth is anticipated across various regions, with North America and Europe currently holding significant market shares. However, emerging markets in Asia-Pacific and Latin America are expected to witness rapid growth due to rising healthcare expenditure and increasing awareness of CKD. The market's future will likely be shaped by factors such as the development of novel biocompatible materials for improved AVF patency, personalized medicine approaches to AVF creation, and the integration of telehealth for remote monitoring and management of AVFs. Regulatory approvals and reimbursement policies in different geographies will also play a crucial role in shaping market dynamics.
Arteriovenous Fistula Devices Market: A Comprehensive Report (2019-2033)
This comprehensive report provides an in-depth analysis of the Arteriovenous Fistula (AVF) Devices market, encompassing market dynamics, growth trends, regional landscapes, product innovations, and key players. The report covers the historical period (2019-2024), base year (2025), and forecast period (2025-2033), offering valuable insights for industry professionals, investors, and stakeholders. The parent market is the vascular access devices market, and the child market is specifically AVF devices. The market size is estimated at xx Million units in 2025.
Arteriovenous Fistula Devices Market Market Dynamics & Structure
This section analyzes the competitive landscape of the AVF devices market, considering market concentration, technological innovation, regulatory landscapes, and market dynamics. The market is moderately fragmented with several key players competing, creating a dynamic environment.
- Market Concentration: The market exhibits a moderately concentrated structure with key players holding significant market share, but also numerous smaller companies contributing to overall market growth. Precise market share percentages are unavailable for the current year but can be estimated. Medtronic, BD, and Fresenius Medical Care are expected to hold the largest shares.
- Technological Innovation: Continuous advancements in materials science, surgical techniques, and device design are driving the market. Innovation focuses on improving AVF maturation rates, reducing complications, and enhancing patient outcomes.
- Regulatory Frameworks: Stringent regulatory approvals (e.g., FDA) and reimbursement policies significantly influence market dynamics. Compliance with these frameworks is crucial for market entry and sustained growth.
- Competitive Substitutes: Other vascular access methods (central venous catheters, arteriovenous grafts) compete with AVF devices, impacting market adoption rates and growth potential.
- End-User Demographics: The increasing prevalence of chronic kidney disease (CKD) is a major driver of market growth, as AVFs are the preferred access method for hemodialysis. The aging global population further contributes to market expansion.
- M&A Trends: Moderate M&A activity is observed, with larger companies acquiring smaller companies to expand their product portfolios and access new technologies. An estimated xx M&A deals occurred within the historical period (2019-2024).
Arteriovenous Fistula Devices Market Growth Trends & Insights
The global AVF devices market is experiencing substantial growth, driven by factors such as the rising prevalence of end-stage renal disease (ESRD), technological advancements, and increasing demand for improved hemodialysis access. The market is estimated to show a CAGR of xx% during the forecast period (2025-2033). This growth is attributed to several key factors, including:
- Rising prevalence of chronic kidney disease (CKD) leading to increased demand for hemodialysis.
- Technological advancements in AVF device design and materials, improving maturation rates and reducing complications.
- Growing awareness among healthcare professionals and patients regarding the benefits of AVFs over other vascular access methods.
- Favorable regulatory landscape in key markets, facilitating market entry for new products.
Market penetration is expected to increase from xx% in 2025 to xx% in 2033, driven by growing adoption of advanced AVF devices in emerging markets.
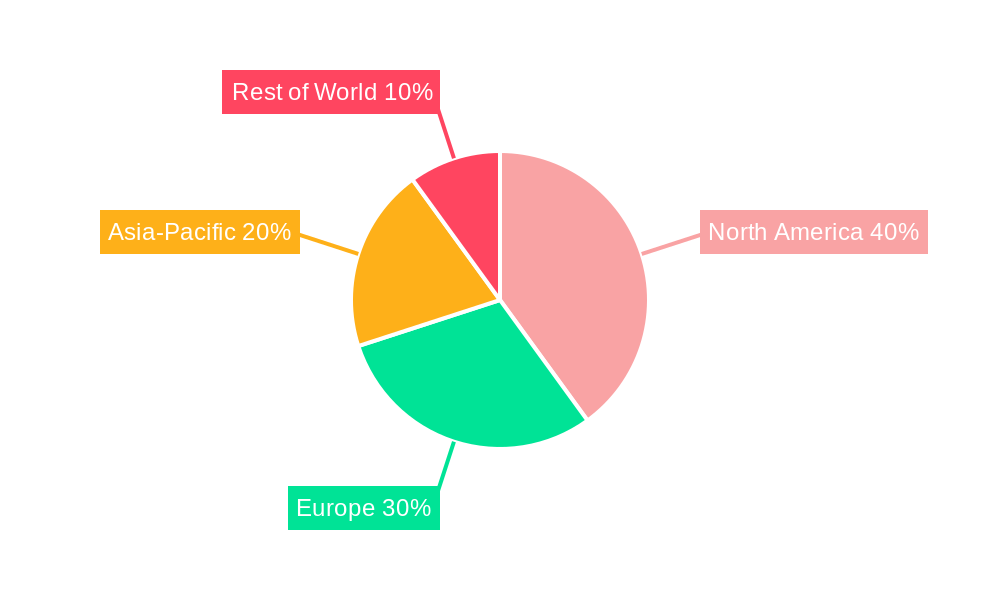
Dominant Regions, Countries, or Segments in Arteriovenous Fistula Devices Market
North America currently dominates the AVF devices market, driven by high prevalence of CKD, advanced healthcare infrastructure, and high adoption rates of advanced technologies. However, the Asia-Pacific region is expected to exhibit the highest growth rate due to increasing prevalence of CKD, rising disposable incomes, and improved healthcare infrastructure.
- North America: High adoption rates, advanced healthcare infrastructure, favorable reimbursement policies, and strong presence of key market players contribute to market dominance.
- Europe: A mature market with steady growth, driven by government initiatives to improve CKD care and aging population.
- Asia-Pacific: High growth potential driven by increasing prevalence of CKD, rising disposable incomes, and expanding healthcare infrastructure in emerging economies like India and China.
Within these regions, specific countries with strong healthcare systems and substantial CKD patient populations will show the greatest market growth.
Arteriovenous Fistula Devices Market Product Landscape
The AVF devices market offers a range of products, including needles, catheters, and other access devices. Innovation focuses on improving cannulation ease, reducing thrombosis risk, and enhancing overall device longevity. Unique selling propositions include improved biocompatibility, enhanced durability, and advanced features for easier access and reduced complications. Technological advancements such as the incorporation of novel materials, specialized coatings, and improved design features contribute to enhanced performance metrics.
Key Drivers, Barriers & Challenges in Arteriovenous Fistula Devices Market
Key Drivers:
- Rising prevalence of chronic kidney disease (CKD) globally.
- Technological advancements leading to improved AVF device performance.
- Increasing preference for AVFs over other vascular access methods.
Key Barriers & Challenges:
- High initial costs associated with AVF creation and maintenance.
- Risk of complications, such as thrombosis, stenosis, and infection.
- Limited reimbursement coverage in certain regions.
- Stringent regulatory requirements for device approval. xx% of new devices fail to achieve regulatory approval.
Emerging Opportunities in Arteriovenous Fistula Devices Market
- Development of innovative AVF devices with improved biocompatibility and reduced complication rates.
- Expansion into untapped markets in developing countries.
- Development of telehealth solutions for remote monitoring of AVF patency.
- Development of personalized medicine approaches to optimize AVF creation and management.
Growth Accelerators in the Arteriovenous Fistula Devices Market Industry
Technological advancements, such as the development of novel materials and improved device designs, are key growth accelerators. Strategic partnerships between device manufacturers and healthcare providers are also crucial for market expansion. Focus on improving patient education and increasing awareness among healthcare professionals can further accelerate growth.
Key Players Shaping the Arteriovenous Fistula Devices Market Market
- Medtronic
- BD
- Teleflex Incorporated
- Fresenius Medical Care AG & Co KGaA
- B Braun SE
- Polymedicure
- NxStage Medical Inc
- Laminate Medical Technologies
- Cook Medical
- Asahi Kasei Medical Co Ltd
- List Not Exhaustive
Notable Milestones in Arteriovenous Fistula Devices Market Sector
- September 2024: XS Innovations secures EUR 1.1 million (USD 1.22 million) in seed funding to develop its Dynamic AVF device, aimed at reducing hemodialysis complications. This signifies increased investment in innovative AVF technologies.
- January 2024: Laminate Medical successfully implants its VasQ device in the US, the first of its kind approved by the FDA, marking a significant advancement in surgical fistula creation. This highlights the market's focus on improving surgical fistula success rates.
In-Depth Arteriovenous Fistula Devices Market Market Outlook
The AVF devices market is poised for continued growth, driven by increasing prevalence of CKD, technological innovation, and expanding healthcare infrastructure globally. Strategic partnerships, product diversification, and focus on emerging markets will be key to unlocking significant growth potential in the coming years. The market is expected to reach xx Million units by 2033, presenting substantial opportunities for market participants.
Arteriovenous Fistula Devices Market Segmentation
-
1. Type
-
1.1. AVF Creation Devices
- 1.1.1. Surgical Instruments
- 1.1.2. Vascular Grafts
- 1.1.3. Angioplasty Balloons
- 1.1.4. Others
-
1.2. AVF Monitoring Devices
- 1.2.1. Doppler Ultrasound
- 1.2.2. Pressure Monitoring Systems
-
1.3. AVF Maintenance Devices
- 1.3.1. Central Venous Catheters
- 1.3.2. Stents
-
1.1. AVF Creation Devices
-
2. End User
- 2.1. Hospitals
- 2.2. Ambulatory Surgical Centers
- 2.3. Dialysis Centers
Arteriovenous Fistula Devices Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
Arteriovenous Fistula Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 7.50% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of End Stage Renal Disease (ESRD); Increasing Focus on Minimally Invasive Procedures and Increasing Aging Population
- 3.3. Market Restrains
- 3.3.1. Increasing Prevalence of End Stage Renal Disease (ESRD); Increasing Focus on Minimally Invasive Procedures and Increasing Aging Population
- 3.4. Market Trends
- 3.4.1. The Vascular Grafts Segment is Expected to Hold a Significant Share During the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Arteriovenous Fistula Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. AVF Creation Devices
- 5.1.1.1. Surgical Instruments
- 5.1.1.2. Vascular Grafts
- 5.1.1.3. Angioplasty Balloons
- 5.1.1.4. Others
- 5.1.2. AVF Monitoring Devices
- 5.1.2.1. Doppler Ultrasound
- 5.1.2.2. Pressure Monitoring Systems
- 5.1.3. AVF Maintenance Devices
- 5.1.3.1. Central Venous Catheters
- 5.1.3.2. Stents
- 5.1.1. AVF Creation Devices
- 5.2. Market Analysis, Insights and Forecast - by End User
- 5.2.1. Hospitals
- 5.2.2. Ambulatory Surgical Centers
- 5.2.3. Dialysis Centers
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia Pacific
- 5.3.4. Middle East and Africa
- 5.3.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. North America Arteriovenous Fistula Devices Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Type
- 6.1.1. AVF Creation Devices
- 6.1.1.1. Surgical Instruments
- 6.1.1.2. Vascular Grafts
- 6.1.1.3. Angioplasty Balloons
- 6.1.1.4. Others
- 6.1.2. AVF Monitoring Devices
- 6.1.2.1. Doppler Ultrasound
- 6.1.2.2. Pressure Monitoring Systems
- 6.1.3. AVF Maintenance Devices
- 6.1.3.1. Central Venous Catheters
- 6.1.3.2. Stents
- 6.1.1. AVF Creation Devices
- 6.2. Market Analysis, Insights and Forecast - by End User
- 6.2.1. Hospitals
- 6.2.2. Ambulatory Surgical Centers
- 6.2.3. Dialysis Centers
- 6.1. Market Analysis, Insights and Forecast - by Type
- 7. Europe Arteriovenous Fistula Devices Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Type
- 7.1.1. AVF Creation Devices
- 7.1.1.1. Surgical Instruments
- 7.1.1.2. Vascular Grafts
- 7.1.1.3. Angioplasty Balloons
- 7.1.1.4. Others
- 7.1.2. AVF Monitoring Devices
- 7.1.2.1. Doppler Ultrasound
- 7.1.2.2. Pressure Monitoring Systems
- 7.1.3. AVF Maintenance Devices
- 7.1.3.1. Central Venous Catheters
- 7.1.3.2. Stents
- 7.1.1. AVF Creation Devices
- 7.2. Market Analysis, Insights and Forecast - by End User
- 7.2.1. Hospitals
- 7.2.2. Ambulatory Surgical Centers
- 7.2.3. Dialysis Centers
- 7.1. Market Analysis, Insights and Forecast - by Type
- 8. Asia Pacific Arteriovenous Fistula Devices Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Type
- 8.1.1. AVF Creation Devices
- 8.1.1.1. Surgical Instruments
- 8.1.1.2. Vascular Grafts
- 8.1.1.3. Angioplasty Balloons
- 8.1.1.4. Others
- 8.1.2. AVF Monitoring Devices
- 8.1.2.1. Doppler Ultrasound
- 8.1.2.2. Pressure Monitoring Systems
- 8.1.3. AVF Maintenance Devices
- 8.1.3.1. Central Venous Catheters
- 8.1.3.2. Stents
- 8.1.1. AVF Creation Devices
- 8.2. Market Analysis, Insights and Forecast - by End User
- 8.2.1. Hospitals
- 8.2.2. Ambulatory Surgical Centers
- 8.2.3. Dialysis Centers
- 8.1. Market Analysis, Insights and Forecast - by Type
- 9. Middle East and Africa Arteriovenous Fistula Devices Market Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Type
- 9.1.1. AVF Creation Devices
- 9.1.1.1. Surgical Instruments
- 9.1.1.2. Vascular Grafts
- 9.1.1.3. Angioplasty Balloons
- 9.1.1.4. Others
- 9.1.2. AVF Monitoring Devices
- 9.1.2.1. Doppler Ultrasound
- 9.1.2.2. Pressure Monitoring Systems
- 9.1.3. AVF Maintenance Devices
- 9.1.3.1. Central Venous Catheters
- 9.1.3.2. Stents
- 9.1.1. AVF Creation Devices
- 9.2. Market Analysis, Insights and Forecast - by End User
- 9.2.1. Hospitals
- 9.2.2. Ambulatory Surgical Centers
- 9.2.3. Dialysis Centers
- 9.1. Market Analysis, Insights and Forecast - by Type
- 10. South America Arteriovenous Fistula Devices Market Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Type
- 10.1.1. AVF Creation Devices
- 10.1.1.1. Surgical Instruments
- 10.1.1.2. Vascular Grafts
- 10.1.1.3. Angioplasty Balloons
- 10.1.1.4. Others
- 10.1.2. AVF Monitoring Devices
- 10.1.2.1. Doppler Ultrasound
- 10.1.2.2. Pressure Monitoring Systems
- 10.1.3. AVF Maintenance Devices
- 10.1.3.1. Central Venous Catheters
- 10.1.3.2. Stents
- 10.1.1. AVF Creation Devices
- 10.2. Market Analysis, Insights and Forecast - by End User
- 10.2.1. Hospitals
- 10.2.2. Ambulatory Surgical Centers
- 10.2.3. Dialysis Centers
- 10.1. Market Analysis, Insights and Forecast - by Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Medtronic
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 BD
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Teleflex Incorporated
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Fresenius Medical Care AG & Co KGaA
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 B Braun SE
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Polymedicure
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 NxStage Medical Inc
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Laminate Medical Technologies
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Cook Medical
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Asahi Kasei Medical Co Ltd*List Not Exhaustive
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.1 Medtronic
List of Figures
- Figure 1: Global Arteriovenous Fistula Devices Market Revenue Breakdown (Million, %) by Region 2024 & 2032
- Figure 2: Global Arteriovenous Fistula Devices Market Volume Breakdown (Billion, %) by Region 2024 & 2032
- Figure 3: North America Arteriovenous Fistula Devices Market Revenue (Million), by Type 2024 & 2032
- Figure 4: North America Arteriovenous Fistula Devices Market Volume (Billion), by Type 2024 & 2032
- Figure 5: North America Arteriovenous Fistula Devices Market Revenue Share (%), by Type 2024 & 2032
- Figure 6: North America Arteriovenous Fistula Devices Market Volume Share (%), by Type 2024 & 2032
- Figure 7: North America Arteriovenous Fistula Devices Market Revenue (Million), by End User 2024 & 2032
- Figure 8: North America Arteriovenous Fistula Devices Market Volume (Billion), by End User 2024 & 2032
- Figure 9: North America Arteriovenous Fistula Devices Market Revenue Share (%), by End User 2024 & 2032
- Figure 10: North America Arteriovenous Fistula Devices Market Volume Share (%), by End User 2024 & 2032
- Figure 11: North America Arteriovenous Fistula Devices Market Revenue (Million), by Country 2024 & 2032
- Figure 12: North America Arteriovenous Fistula Devices Market Volume (Billion), by Country 2024 & 2032
- Figure 13: North America Arteriovenous Fistula Devices Market Revenue Share (%), by Country 2024 & 2032
- Figure 14: North America Arteriovenous Fistula Devices Market Volume Share (%), by Country 2024 & 2032
- Figure 15: Europe Arteriovenous Fistula Devices Market Revenue (Million), by Type 2024 & 2032
- Figure 16: Europe Arteriovenous Fistula Devices Market Volume (Billion), by Type 2024 & 2032
- Figure 17: Europe Arteriovenous Fistula Devices Market Revenue Share (%), by Type 2024 & 2032
- Figure 18: Europe Arteriovenous Fistula Devices Market Volume Share (%), by Type 2024 & 2032
- Figure 19: Europe Arteriovenous Fistula Devices Market Revenue (Million), by End User 2024 & 2032
- Figure 20: Europe Arteriovenous Fistula Devices Market Volume (Billion), by End User 2024 & 2032
- Figure 21: Europe Arteriovenous Fistula Devices Market Revenue Share (%), by End User 2024 & 2032
- Figure 22: Europe Arteriovenous Fistula Devices Market Volume Share (%), by End User 2024 & 2032
- Figure 23: Europe Arteriovenous Fistula Devices Market Revenue (Million), by Country 2024 & 2032
- Figure 24: Europe Arteriovenous Fistula Devices Market Volume (Billion), by Country 2024 & 2032
- Figure 25: Europe Arteriovenous Fistula Devices Market Revenue Share (%), by Country 2024 & 2032
- Figure 26: Europe Arteriovenous Fistula Devices Market Volume Share (%), by Country 2024 & 2032
- Figure 27: Asia Pacific Arteriovenous Fistula Devices Market Revenue (Million), by Type 2024 & 2032
- Figure 28: Asia Pacific Arteriovenous Fistula Devices Market Volume (Billion), by Type 2024 & 2032
- Figure 29: Asia Pacific Arteriovenous Fistula Devices Market Revenue Share (%), by Type 2024 & 2032
- Figure 30: Asia Pacific Arteriovenous Fistula Devices Market Volume Share (%), by Type 2024 & 2032
- Figure 31: Asia Pacific Arteriovenous Fistula Devices Market Revenue (Million), by End User 2024 & 2032
- Figure 32: Asia Pacific Arteriovenous Fistula Devices Market Volume (Billion), by End User 2024 & 2032
- Figure 33: Asia Pacific Arteriovenous Fistula Devices Market Revenue Share (%), by End User 2024 & 2032
- Figure 34: Asia Pacific Arteriovenous Fistula Devices Market Volume Share (%), by End User 2024 & 2032
- Figure 35: Asia Pacific Arteriovenous Fistula Devices Market Revenue (Million), by Country 2024 & 2032
- Figure 36: Asia Pacific Arteriovenous Fistula Devices Market Volume (Billion), by Country 2024 & 2032
- Figure 37: Asia Pacific Arteriovenous Fistula Devices Market Revenue Share (%), by Country 2024 & 2032
- Figure 38: Asia Pacific Arteriovenous Fistula Devices Market Volume Share (%), by Country 2024 & 2032
- Figure 39: Middle East and Africa Arteriovenous Fistula Devices Market Revenue (Million), by Type 2024 & 2032
- Figure 40: Middle East and Africa Arteriovenous Fistula Devices Market Volume (Billion), by Type 2024 & 2032
- Figure 41: Middle East and Africa Arteriovenous Fistula Devices Market Revenue Share (%), by Type 2024 & 2032
- Figure 42: Middle East and Africa Arteriovenous Fistula Devices Market Volume Share (%), by Type 2024 & 2032
- Figure 43: Middle East and Africa Arteriovenous Fistula Devices Market Revenue (Million), by End User 2024 & 2032
- Figure 44: Middle East and Africa Arteriovenous Fistula Devices Market Volume (Billion), by End User 2024 & 2032
- Figure 45: Middle East and Africa Arteriovenous Fistula Devices Market Revenue Share (%), by End User 2024 & 2032
- Figure 46: Middle East and Africa Arteriovenous Fistula Devices Market Volume Share (%), by End User 2024 & 2032
- Figure 47: Middle East and Africa Arteriovenous Fistula Devices Market Revenue (Million), by Country 2024 & 2032
- Figure 48: Middle East and Africa Arteriovenous Fistula Devices Market Volume (Billion), by Country 2024 & 2032
- Figure 49: Middle East and Africa Arteriovenous Fistula Devices Market Revenue Share (%), by Country 2024 & 2032
- Figure 50: Middle East and Africa Arteriovenous Fistula Devices Market Volume Share (%), by Country 2024 & 2032
- Figure 51: South America Arteriovenous Fistula Devices Market Revenue (Million), by Type 2024 & 2032
- Figure 52: South America Arteriovenous Fistula Devices Market Volume (Billion), by Type 2024 & 2032
- Figure 53: South America Arteriovenous Fistula Devices Market Revenue Share (%), by Type 2024 & 2032
- Figure 54: South America Arteriovenous Fistula Devices Market Volume Share (%), by Type 2024 & 2032
- Figure 55: South America Arteriovenous Fistula Devices Market Revenue (Million), by End User 2024 & 2032
- Figure 56: South America Arteriovenous Fistula Devices Market Volume (Billion), by End User 2024 & 2032
- Figure 57: South America Arteriovenous Fistula Devices Market Revenue Share (%), by End User 2024 & 2032
- Figure 58: South America Arteriovenous Fistula Devices Market Volume Share (%), by End User 2024 & 2032
- Figure 59: South America Arteriovenous Fistula Devices Market Revenue (Million), by Country 2024 & 2032
- Figure 60: South America Arteriovenous Fistula Devices Market Volume (Billion), by Country 2024 & 2032
- Figure 61: South America Arteriovenous Fistula Devices Market Revenue Share (%), by Country 2024 & 2032
- Figure 62: South America Arteriovenous Fistula Devices Market Volume Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Region 2019 & 2032
- Table 3: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 4: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Type 2019 & 2032
- Table 5: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by End User 2019 & 2032
- Table 6: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by End User 2019 & 2032
- Table 7: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 8: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Region 2019 & 2032
- Table 9: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 10: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Type 2019 & 2032
- Table 11: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by End User 2019 & 2032
- Table 12: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by End User 2019 & 2032
- Table 13: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 14: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Country 2019 & 2032
- Table 15: United States Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: United States Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 17: Canada Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Canada Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 19: Mexico Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Mexico Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 21: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 22: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Type 2019 & 2032
- Table 23: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by End User 2019 & 2032
- Table 24: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by End User 2019 & 2032
- Table 25: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 26: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Country 2019 & 2032
- Table 27: Germany Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 28: Germany Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 29: United Kingdom Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: United Kingdom Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 31: France Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 32: France Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 33: Italy Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 34: Italy Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 35: Spain Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 36: Spain Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 37: Rest of Europe Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 38: Rest of Europe Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 39: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 40: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Type 2019 & 2032
- Table 41: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by End User 2019 & 2032
- Table 42: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by End User 2019 & 2032
- Table 43: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 44: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Country 2019 & 2032
- Table 45: China Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 46: China Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 47: Japan Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 48: Japan Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 49: India Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 50: India Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 51: Australia Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 52: Australia Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 53: South Korea Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 54: South Korea Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 55: Rest of Asia Pacific Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 56: Rest of Asia Pacific Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 57: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 58: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Type 2019 & 2032
- Table 59: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by End User 2019 & 2032
- Table 60: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by End User 2019 & 2032
- Table 61: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 62: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Country 2019 & 2032
- Table 63: GCC Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 64: GCC Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 65: South Africa Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 66: South Africa Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 67: Rest of Middle East and Africa Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 68: Rest of Middle East and Africa Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 69: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 70: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Type 2019 & 2032
- Table 71: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by End User 2019 & 2032
- Table 72: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by End User 2019 & 2032
- Table 73: Global Arteriovenous Fistula Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 74: Global Arteriovenous Fistula Devices Market Volume Billion Forecast, by Country 2019 & 2032
- Table 75: Brazil Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 76: Brazil Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 77: Argentina Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 78: Argentina Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
- Table 79: Rest of South America Arteriovenous Fistula Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 80: Rest of South America Arteriovenous Fistula Devices Market Volume (Billion) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Arteriovenous Fistula Devices Market?
The projected CAGR is approximately 7.50%.
2. Which companies are prominent players in the Arteriovenous Fistula Devices Market?
Key companies in the market include Medtronic, BD, Teleflex Incorporated, Fresenius Medical Care AG & Co KGaA, B Braun SE, Polymedicure, NxStage Medical Inc, Laminate Medical Technologies, Cook Medical, Asahi Kasei Medical Co Ltd*List Not Exhaustive.
3. What are the main segments of the Arteriovenous Fistula Devices Market?
The market segments include Type, End User.
4. Can you provide details about the market size?
The market size is estimated to be USD 1.07 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of End Stage Renal Disease (ESRD); Increasing Focus on Minimally Invasive Procedures and Increasing Aging Population.
6. What are the notable trends driving market growth?
The Vascular Grafts Segment is Expected to Hold a Significant Share During the Forecast Period.
7. Are there any restraints impacting market growth?
Increasing Prevalence of End Stage Renal Disease (ESRD); Increasing Focus on Minimally Invasive Procedures and Increasing Aging Population.
8. Can you provide examples of recent developments in the market?
September 2024: XS Innovations, a spinout from Leiden University Medical Center (LUMC) and Delft University of Technology (TU Delft), closed a seed funding round, raising EUR 1.1 million (USD 1.22 million). These funds will expedite the development of XS Innovations’ Dynamic AVF, a vascular access device aimed at mitigating common complications linked to hemodialysis.January 2024: Laminate Medical successfully implanted its VasQ device for the first time in the United States, with the procedure conducted by Ari Kramer at Spartanburg Regional Hospital in Spartanburg, United States. The VasQ technology received approval from the United States Food and Drug Administration (FDA) in September 2023. Notably, it stands as the sole product of its kind currently approved on the market, specifically engineered to enhance the success of surgical fistulas right from their inception.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in Billion.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Arteriovenous Fistula Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Arteriovenous Fistula Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Arteriovenous Fistula Devices Market?
To stay informed about further developments, trends, and reports in the Arteriovenous Fistula Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


