Key Insights
The Brazilian ophthalmic devices market is poised for significant expansion, projecting a Compound Annual Growth Rate (CAGR) of 5.12%. This robust growth, from a base year of 2025 with a market size of 511.1 million, is driven by an aging demographic experiencing a rise in age-related eye conditions. Increased vision correction awareness and enhanced healthcare accessibility, especially in urban centers, are further propelling market dynamics. Technological innovations in surgical and diagnostic equipment, enabling less invasive procedures and superior accuracy, are also key contributors to demand. The market is segmented by device type, with surgical and diagnostic/monitoring devices commanding substantial shares. Vision correction devices, including refractive surgery technologies and contact lenses, form another prominent segment. Key industry leaders are actively engaged in research, development, and distribution network expansion to meet escalating demand.
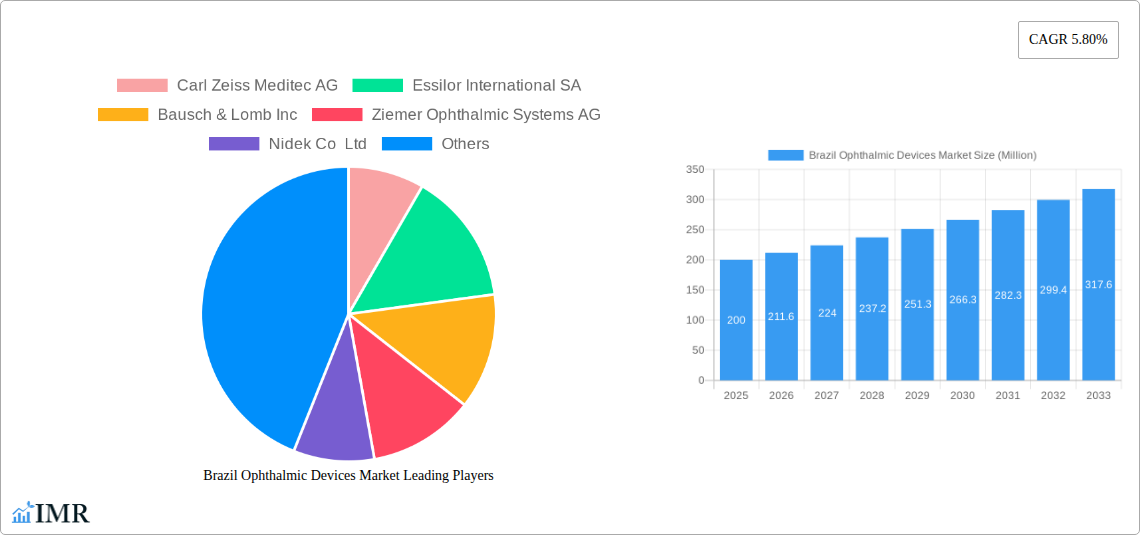
Brazil Ophthalmic Devices Market Market Size (In Million)

Despite strong growth prospects, challenges such as cost accessibility for advanced technologies and regional healthcare infrastructure disparities persist. However, government initiatives focused on healthcare affordability and access, alongside increasing private investment, are expected to alleviate these constraints. This dynamic sector presents considerable opportunities for ophthalmic device manufacturers and healthcare providers prioritizing innovation, affordability, and accessibility. The forecast period, 2025-2033, indicates substantial market growth.
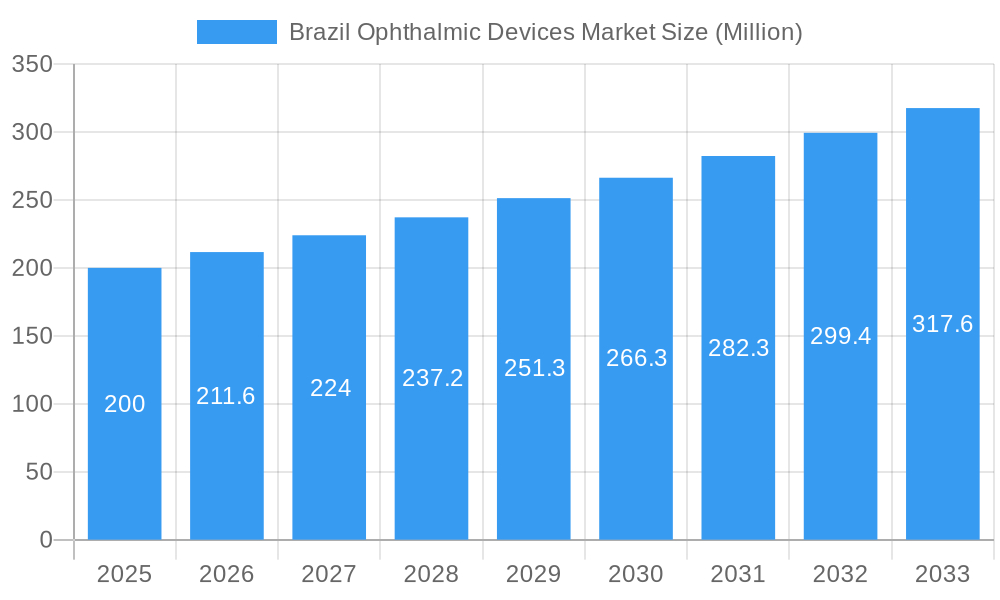
Brazil Ophthalmic Devices Market Company Market Share

Brazil Ophthalmic Devices Market: A Comprehensive Report (2019-2033)
This in-depth report provides a comprehensive analysis of the Brazil ophthalmic devices market, offering invaluable insights for industry professionals, investors, and strategic decision-makers. The report covers the period 2019-2033, with a focus on the base year 2025 and a forecast period of 2025-2033. The market is segmented by device type: Surgical Devices, Diagnostic and Monitoring Devices, and Vision Correction Devices. Key players such as Carl Zeiss Meditec AG, Essilor International SA, Bausch & Lomb Inc, Ziemer Ophthalmic Systems AG, Nidek Co Ltd, Johnson & Johnson, Topcon Corporation, and Alcon Inc are analyzed, though the list is not exhaustive. The total market value in 2025 is estimated at XX Million units.
Brazil Ophthalmic Devices Market Market Dynamics & Structure
This section analyzes the competitive landscape, technological advancements, regulatory influences, and market trends within the Brazilian ophthalmic devices sector. The market exhibits a moderately concentrated structure, with a few major players holding significant market share. Technological innovation is a key driver, fueled by increasing demand for minimally invasive procedures and improved diagnostic capabilities. Regulatory frameworks, while generally supportive of medical device innovation, present certain hurdles for market entry and product approval. The market witnesses ongoing mergers and acquisitions (M&A) activity, shaping the competitive dynamics and driving consolidation. Substitutes for specific devices are limited, primarily due to the specialized nature of ophthalmic procedures.
- Market Concentration: XX% market share held by top 5 players in 2025.
- Technological Innovation: Focus on minimally invasive surgery, AI-powered diagnostics, and smart contact lenses.
- Regulatory Framework: ANVISA (Agência Nacional de Vigilância Sanitária) plays a crucial role in approvals and market access.
- M&A Activity: XX M&A deals recorded between 2019 and 2024, leading to increased market consolidation.
- End-User Demographics: Aging population and rising prevalence of ophthalmic diseases are driving market growth. XX% of the population aged 65+ experience age-related vision impairment.
- Competitive Product Substitutes: Limited substitutes exist due to the specialized nature of treatments.
Brazil Ophthalmic Devices Market Growth Trends & Insights
The Brazilian ophthalmic devices market has shown consistent growth from 2019 to 2024, with a Compound Annual Growth Rate (CAGR) of XX% during the historical period. This growth is attributed to several factors, including the increasing prevalence of ophthalmic diseases, rising disposable incomes, and improved healthcare infrastructure in key regions. Adoption rates of advanced technologies are also increasing, driven by the desire for improved treatment outcomes and patient comfort. Technological disruptions, such as the introduction of innovative surgical techniques and advanced diagnostic tools, are significantly influencing market growth. Shifts in consumer behavior, marked by increased awareness of ophthalmic health and a preference for minimally invasive procedures, are further boosting market expansion. Market penetration of advanced devices remains relatively low, presenting significant growth opportunities.
The market size is projected to reach XX Million units by 2033, indicating substantial growth potential.
Dominant Regions, Countries, or Segments in Brazil Ophthalmic Devices Market
The Southeast region of Brazil dominates the ophthalmic devices market, driven by higher population density, improved healthcare infrastructure, and increased economic activity. São Paulo, in particular, is a major market hub. Within the device segments, Surgical Devices hold the largest market share, followed by Diagnostic and Monitoring Devices and Vision Correction Devices. The growth of surgical devices is fueled by an increasing number of cataract surgeries and other refractive procedures. The diagnostic segment's growth is spurred by rising demand for advanced diagnostic tools enabling early detection and precise treatment planning.
- Southeast Region Dominance: Higher population density, better healthcare infrastructure, and strong economic activity.
- Surgical Devices: Largest market segment due to rising cataract and refractive surgery volumes.
- Diagnostic and Monitoring Devices: Strong growth due to increased demand for advanced diagnostic technology.
- Vision Correction Devices: Growth propelled by increased awareness of vision correction options and rising disposable incomes.
Brazil Ophthalmic Devices Market Product Landscape
The Brazilian ophthalmic devices market showcases a diverse range of products, characterized by ongoing innovation and technological advancements. Surgical devices, for example, include advanced phacoemulsification systems and laser systems for refractive surgery. Diagnostic tools incorporate state-of-the-art imaging technologies, while vision correction devices range from traditional eyeglasses to sophisticated contact lenses and intraocular lenses (IOLs). These products are often differentiated by their unique selling propositions (USPs), such as enhanced precision, improved safety features, and increased patient comfort. Technological advancements consistently improve the precision, efficiency, and effectiveness of these devices.
Key Drivers, Barriers & Challenges in Brazil Ophthalmic Devices Market
Key Drivers:
- Rising prevalence of age-related eye diseases.
- Increasing disposable incomes and health insurance coverage.
- Technological advancements leading to better treatment outcomes.
- Government initiatives to improve healthcare infrastructure.
Key Barriers and Challenges:
- High cost of advanced devices limits accessibility in certain segments of the population.
- Complex regulatory approval processes can slow down market entry for new products.
- Fluctuations in the Brazilian economy can impact healthcare spending and device sales. The impact of economic instability reduced sales by an estimated XX% in 2022.
Emerging Opportunities in Brazil Ophthalmic Devices Market
Untapped markets exist in underserved regions of Brazil. Innovative applications of telemedicine and AI-powered diagnostics are emerging. Growing consumer preference for minimally invasive procedures and personalized treatments creates new market opportunities.
Growth Accelerators in the Brazil Ophthalmic Devices Market Industry
Technological breakthroughs in areas such as AI-powered diagnostics, minimally invasive surgical techniques, and smart contact lenses will drive market growth. Strategic partnerships between domestic and international companies are accelerating market expansion. Targeted marketing campaigns focusing on patient education and raising awareness of vision health are crucial for long-term market growth.
Key Players Shaping the Brazil Ophthalmic Devices Market Market
Notable Milestones in Brazil Ophthalmic Devices Market Sector
- July 2021: Johnson & Johnson Vision launched the VERITAS Vision System, a next-generation phacoemulsification system.
- July 2021: CooperVision partnered with Plastic Bank to make its clariti 1-day lenses net plastic-neutral in the US, with implications for its Brazilian operations.
In-Depth Brazil Ophthalmic Devices Market Market Outlook
The Brazilian ophthalmic devices market presents significant long-term growth potential. Continued technological innovation, increasing healthcare spending, and a growing aging population will drive market expansion. Strategic partnerships, focused on market penetration and enhancing access to advanced technologies in underserved areas, will be crucial to realizing this potential. The market is poised for continued growth, exceeding XX Million units by 2033.
Brazil Ophthalmic Devices Market Segmentation
-
1. Devices
-
1.1. Surgical Devices
- 1.1.1. Glaucoma Drainage Devices
- 1.1.2. Glaucoma Stents and Implants
- 1.1.3. Intraocular Lenses
- 1.1.4. Lasers
- 1.1.5. Other Surgical Devices
-
1.2. Diagnostic and Monitoring Devices
- 1.2.1. Autorefractors and Keratometers
- 1.2.2. Corneal Topography Systems
- 1.2.3. Ophthalmic Ultrasound Imaging Systems
- 1.2.4. Ophthalmoscopes
- 1.2.5. Optical Coherence Tomography Scanners
- 1.2.6. Other Diagnostic and Monitoring Devices
-
1.3. Vision Correction Devices
- 1.3.1. Spectacles
- 1.3.2. Contact Lenses
-
1.1. Surgical Devices
Brazil Ophthalmic Devices Market Segmentation By Geography
- 1. Brazil
Brazil Ophthalmic Devices Market Regional Market Share

Geographic Coverage of Brazil Ophthalmic Devices Market
Brazil Ophthalmic Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.12% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Eye Disease; Increasing Geriatric Population
- 3.3. Market Restrains
- 3.3.1. Shortage of Skilled Professionals
- 3.4. Market Trends
- 3.4.1. Contact Lenses Segment is Expected to Witness Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Brazil Ophthalmic Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Devices
- 5.1.1. Surgical Devices
- 5.1.1.1. Glaucoma Drainage Devices
- 5.1.1.2. Glaucoma Stents and Implants
- 5.1.1.3. Intraocular Lenses
- 5.1.1.4. Lasers
- 5.1.1.5. Other Surgical Devices
- 5.1.2. Diagnostic and Monitoring Devices
- 5.1.2.1. Autorefractors and Keratometers
- 5.1.2.2. Corneal Topography Systems
- 5.1.2.3. Ophthalmic Ultrasound Imaging Systems
- 5.1.2.4. Ophthalmoscopes
- 5.1.2.5. Optical Coherence Tomography Scanners
- 5.1.2.6. Other Diagnostic and Monitoring Devices
- 5.1.3. Vision Correction Devices
- 5.1.3.1. Spectacles
- 5.1.3.2. Contact Lenses
- 5.1.1. Surgical Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Brazil
- 5.1. Market Analysis, Insights and Forecast - by Devices
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Carl Zeiss Meditec AG
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Essilor International SA
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Bausch & Lomb Inc
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Ziemer Ophthalmic Systems AG
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Nidek Co Ltd
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Johnson & Johnson
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Topcon Corporation
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Alcon Inc *List Not Exhaustive
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.1 Carl Zeiss Meditec AG
List of Figures
- Figure 1: Brazil Ophthalmic Devices Market Revenue Breakdown (million, %) by Product 2025 & 2033
- Figure 2: Brazil Ophthalmic Devices Market Share (%) by Company 2025
List of Tables
- Table 1: Brazil Ophthalmic Devices Market Revenue million Forecast, by Devices 2020 & 2033
- Table 2: Brazil Ophthalmic Devices Market Revenue million Forecast, by Region 2020 & 2033
- Table 3: Brazil Ophthalmic Devices Market Revenue million Forecast, by Devices 2020 & 2033
- Table 4: Brazil Ophthalmic Devices Market Revenue million Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Brazil Ophthalmic Devices Market?
The projected CAGR is approximately 5.12%.
2. Which companies are prominent players in the Brazil Ophthalmic Devices Market?
Key companies in the market include Carl Zeiss Meditec AG, Essilor International SA, Bausch & Lomb Inc, Ziemer Ophthalmic Systems AG, Nidek Co Ltd, Johnson & Johnson, Topcon Corporation, Alcon Inc *List Not Exhaustive.
3. What are the main segments of the Brazil Ophthalmic Devices Market?
The market segments include Devices.
4. Can you provide details about the market size?
The market size is estimated to be USD 511.1 million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Eye Disease; Increasing Geriatric Population.
6. What are the notable trends driving market growth?
Contact Lenses Segment is Expected to Witness Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
Shortage of Skilled Professionals.
8. Can you provide examples of recent developments in the market?
In July 2021, Johnson & Johnson Vision announced the global availability of the VERITAS Vision System, next-generation phacoemulsification (phaco) system designed to address three critical areas: patient safety, surgeon efficiency, and comfort.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Brazil Ophthalmic Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Brazil Ophthalmic Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Brazil Ophthalmic Devices Market?
To stay informed about further developments, trends, and reports in the Brazil Ophthalmic Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
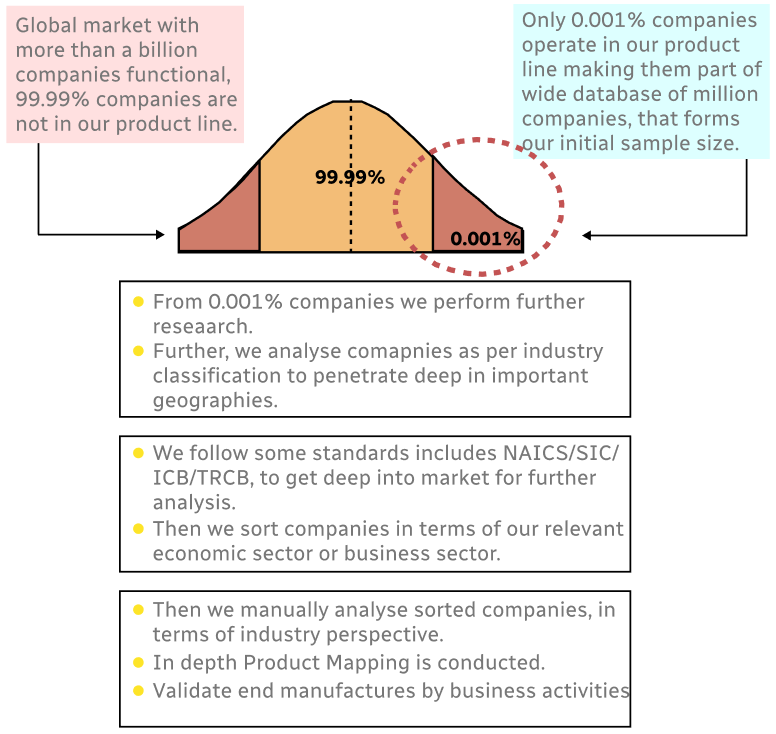
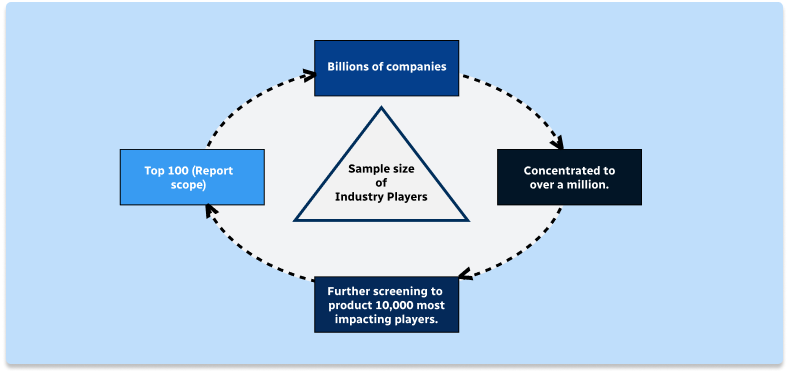
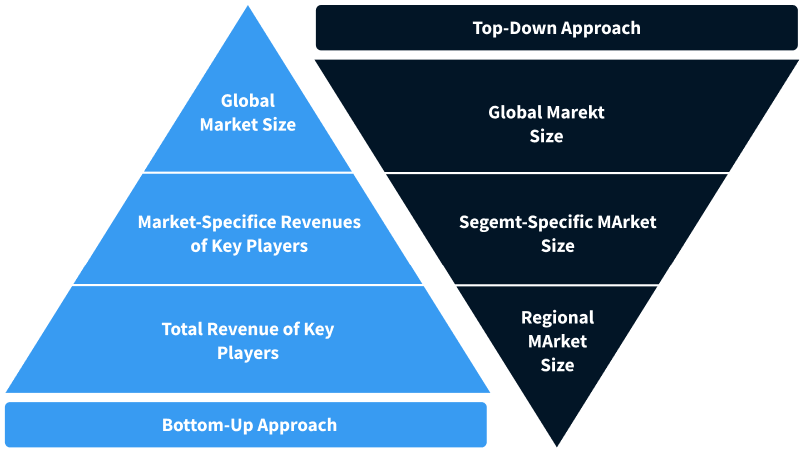
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
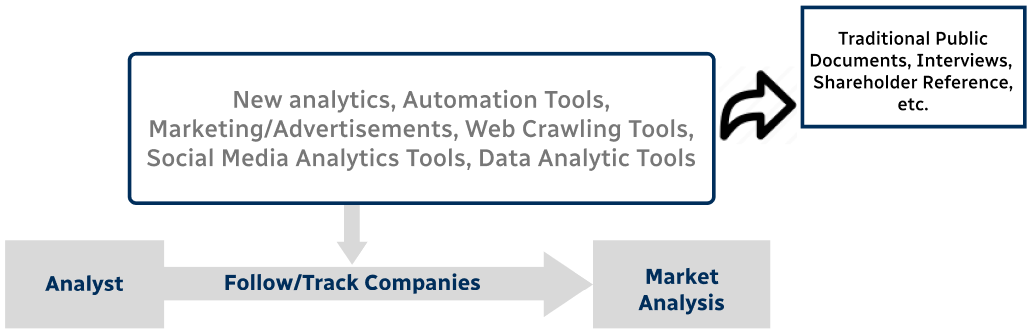
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


