Key Insights
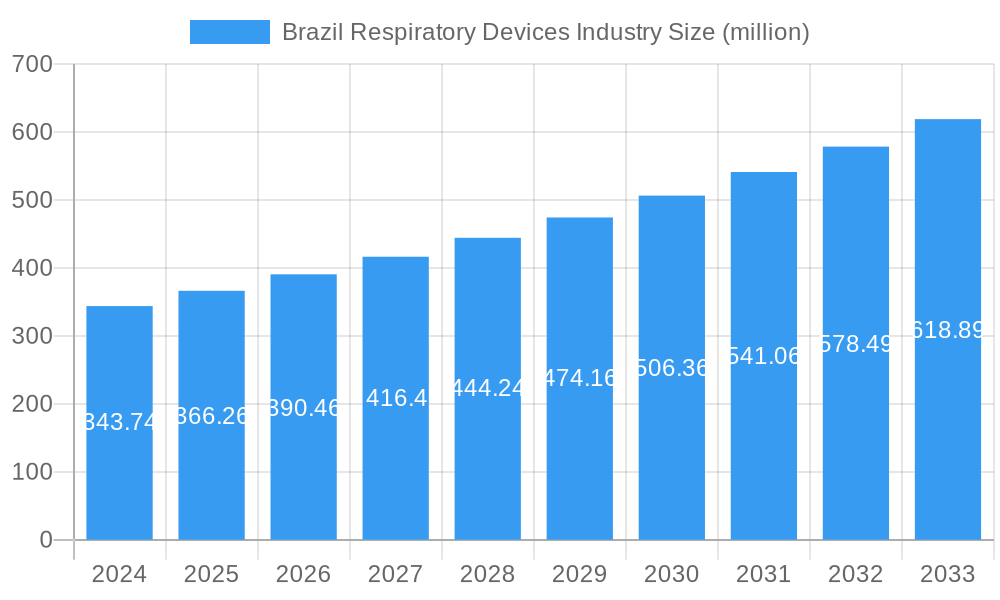
The Brazilian respiratory devices market is poised for significant expansion, projected to reach $343.74 million by 2024, with a robust CAGR of 6.5% anticipated through 2033. This growth is primarily fueled by the escalating prevalence of respiratory ailments such as asthma, COPD, and sleep apnea, exacerbated by rising air pollution, an aging population, and lifestyle-related factors. The increasing adoption of advanced diagnostic and monitoring devices like spirometers and pulse oximeters, alongside therapeutic solutions including CPAP and BiPAP machines, is a key driver. Furthermore, a growing awareness among healthcare providers and patients regarding the importance of early diagnosis and effective management of respiratory conditions contributes to market momentum. The Brazilian government's focus on improving healthcare infrastructure and expanding access to essential medical equipment further bolsters this upward trajectory.
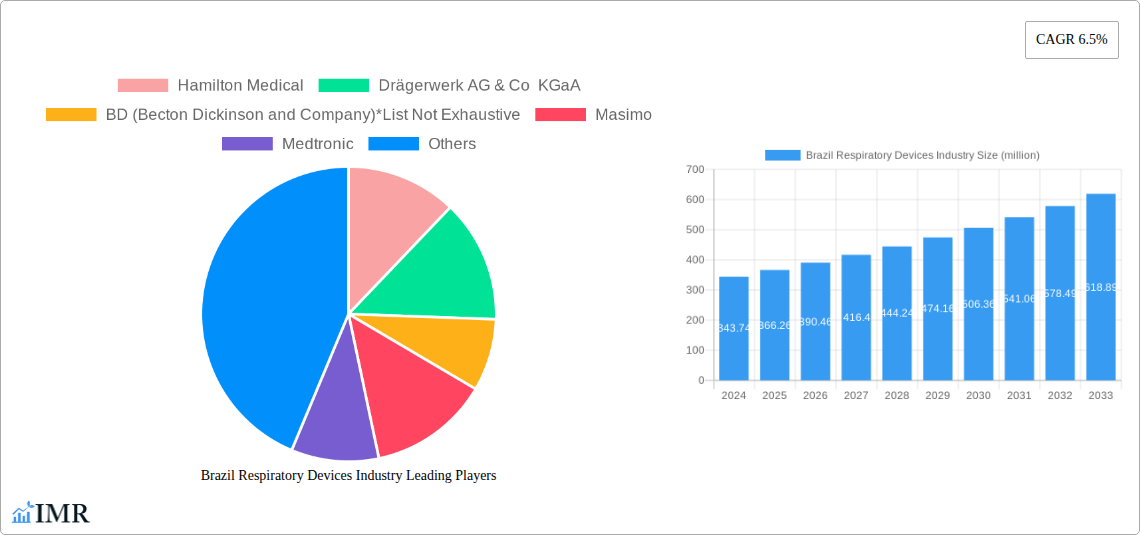
Brazil Respiratory Devices Industry Market Size (In Million)

The market segmentation reveals a strong demand across both diagnostic and monitoring devices, and therapeutic devices, with disposables also playing a crucial role in patient care. Key players like Philips, Medtronic, and ResMed are actively investing in research and development to introduce innovative products tailored to the specific needs of the Brazilian population. Trends such as the increasing demand for home-use respiratory devices, driven by convenience and cost-effectiveness, and the integration of digital technologies for remote patient monitoring, are shaping the market landscape. While the market is characterized by increasing competition and stringent regulatory approvals, the substantial unmet need for quality respiratory care in Brazil presents significant opportunities for sustained growth and innovation in the coming years.
Brazil Respiratory Devices Industry Company Market Share

This comprehensive report provides an in-depth analysis of the Brazil respiratory devices industry, offering critical insights into its market dynamics, structure, and future growth trajectory. We meticulously examine the competitive landscape, technological advancements, and the evolving regulatory environment to equip industry stakeholders with actionable intelligence. This report covers the study period of 2019–2033, with 2025 as the base and estimated year, and a forecast period from 2025–2033, building upon historical data from 2019–2024. All unit values are presented in million units.
Brazil Respiratory Devices Industry Market Dynamics & Structure
The Brazil respiratory devices industry is characterized by a moderately concentrated market, with a few key players holding significant market share. However, the landscape is dynamic, driven by continuous technological innovation and an increasing demand for advanced diagnostic and therapeutic solutions. Regulatory frameworks, particularly those established by ANVISA, play a pivotal role in shaping market entry and product compliance, influencing the adoption of new technologies and the overall competitive environment. The presence of competitive product substitutes, ranging from basic home-use devices to sophisticated hospital-grade equipment, necessitates strategic differentiation and value-driven offerings. End-user demographics, including an aging population and a rising prevalence of respiratory ailments, are key drivers of market growth. Mergers and acquisitions (M&A) activity, while not as pronounced as in more mature markets, is anticipated to increase as companies seek to expand their portfolios and market reach within Brazil.
- Market Concentration: Dominated by a blend of multinational corporations and emerging local players.
- Technological Innovation: Driven by advancements in AI, miniaturization, and connected health solutions for respiratory care.
- Regulatory Framework: ANVISA's evolving regulations, such as RDC 665/2022, are crucial for market access and product quality.
- Competitive Product Substitutes: A broad spectrum of devices catering to diverse needs and price points.
- End-User Demographics: Growing elderly population and increased incidence of COPD, asthma, and sleep apnea are fueling demand.
- M&A Trends: Strategic acquisitions and partnerships are expected to consolidate market share and introduce new technologies.
Brazil Respiratory Devices Industry Growth Trends & Insights
The Brazil respiratory devices industry is poised for significant expansion, fueled by a confluence of factors including increasing healthcare expenditure, rising awareness of respiratory health, and a growing burden of chronic respiratory diseases. The market size is projected to witness a robust Compound Annual Growth Rate (CAGR) throughout the forecast period, driven by higher adoption rates of both diagnostic and therapeutic devices. Technological disruptions, such as the integration of AI in diagnostic tools for early detection and personalized treatment plans, along with advancements in portable and connected therapeutic devices for enhanced patient compliance and remote monitoring, are reshaping consumer behavior and market demands. The shift towards home healthcare solutions and preventative respiratory care is also a major trend, further accelerating market penetration. The increasing accessibility of advanced medical devices, coupled with supportive government initiatives aimed at improving public health infrastructure, will continue to bolster market growth. Consumer preferences are leaning towards user-friendly, efficient, and cost-effective respiratory solutions, leading manufacturers to innovate and adapt their product offerings to meet these evolving needs. The estimated market size in 2025 is XX million units, with projections indicating substantial growth by 2033.
Dominant Regions, Countries, or Segments in Brazil Respiratory Devices Industry
Within the Brazil respiratory devices industry, the Therapeutic Devices segment stands out as a primary growth engine, driven by the increasing prevalence of chronic respiratory conditions such as Chronic Obstructive Pulmonary Disease (COPD), asthma, and sleep apnea. This segment encompasses a wide array of essential products including CPAP devices, BiPAP devices, humidifiers, nebulizers, oxygen concentrators, ventilators, and inhalers, all of which are experiencing heightened demand. The market dominance of therapeutic devices is underpinned by several key drivers.
- High Disease Prevalence: Brazil faces a significant burden of respiratory illnesses, necessitating continuous and often long-term therapeutic interventions. This drives consistent demand for devices like CPAP and BiPAP machines for sleep disorders, and oxygen concentrators for chronic lung conditions.
- Aging Population: The demographic shift towards an older population in Brazil contributes significantly to the demand for respiratory support devices, as age is a critical risk factor for many respiratory ailments.
- Government Healthcare Initiatives: Investments in public health infrastructure and programs aimed at managing chronic diseases are expanding access to therapeutic respiratory devices, particularly in underserved regions.
- Technological Advancements: Innovations in therapeutic devices, such as quieter and more portable CPAP machines, advanced ventilator functionalities, and more efficient nebulizers, are enhancing patient comfort and treatment outcomes, thereby driving adoption.
- Home Healthcare Growth: A burgeoning trend towards home-based care for chronic conditions has boosted the demand for user-friendly and effective therapeutic devices that can be managed outside of hospital settings. The market share for therapeutic devices is estimated to be around XX% in 2025, with significant growth potential projected through 2033.
Among the Diagnostic and Monitoring Devices, Pulse Oximeters and Sleep Test Devices are experiencing rapid growth. Pulse oximeters are essential for non-invasive monitoring of blood oxygen saturation, critical for various acute and chronic respiratory conditions, and their demand has surged due to their utility in both clinical and home settings. Sleep test devices, including polysomnography and ambulatory monitoring systems, are vital for diagnosing sleep disorders, which are increasingly recognized as significant public health issues. The estimated market share for Diagnostic and Monitoring Devices in 2025 is XX%, with Pulse Oximeters and Sleep Test Devices contributing a substantial portion of this value.
The Disposables segment, including masks and breathing circuits, demonstrates consistent demand, directly correlating with the usage of therapeutic and diagnostic devices. While not a standalone growth driver, its expansion is intrinsically linked to the overall market growth of respiratory care equipment. The estimated market share for Disposables in 2025 is XX%.
The dominance of these segments is further amplified by Brazil's large population and the increasing focus on public health awareness and preventative care strategies. Economic policies that encourage healthcare infrastructure development and the adoption of advanced medical technologies also play a crucial role in sustaining and accelerating the growth of these dominant segments.
Brazil Respiratory Devices Industry Product Landscape
The Brazil respiratory devices industry is witnessing a surge in product innovations focusing on enhanced portability, connectivity, and user-friendliness. Manufacturers are developing advanced CPAP and BiPAP devices with intelligent pressure adjustments and integrated humidification for superior patient comfort and compliance. In the diagnostic realm, miniaturized pulse oximeters and capnographs are enabling real-time, non-invasive monitoring in diverse clinical settings, including home care. Oxygen concentrators are becoming more energy-efficient and quieter, catering to the growing demand for home oxygen therapy. Furthermore, the integration of IoT capabilities allows for remote patient monitoring and data analytics, paving the way for personalized respiratory care and early intervention. These advancements not only improve treatment efficacy but also address the growing need for accessible and convenient respiratory solutions in Brazil.
Key Drivers, Barriers & Challenges in Brazil Respiratory Devices Industry
Key Drivers:
- Rising prevalence of respiratory diseases: Increasing incidence of COPD, asthma, and sleep apnea drives demand for therapeutic and diagnostic devices.
- Aging population: The growing elderly demographic is more susceptible to respiratory ailments, boosting market needs.
- Technological advancements: Innovations in connected devices, AI-powered diagnostics, and miniaturization enhance product utility and adoption.
- Growing healthcare expenditure: Increased investment in healthcare infrastructure and services supports the adoption of advanced respiratory equipment.
Barriers & Challenges:
- High cost of advanced devices: The price of sophisticated respiratory equipment can be a barrier to widespread adoption, especially for a significant portion of the population.
- Regulatory hurdles: Navigating ANVISA's evolving regulatory landscape, while ensuring compliance, can be complex and time-consuming for manufacturers.
- Supply chain disruptions: Global and local supply chain vulnerabilities can impact the availability and timely delivery of essential components and finished products.
- Limited reimbursement policies: Inconsistent or inadequate reimbursement policies for certain respiratory devices can hinder market penetration and accessibility.
- Awareness and education gaps: A lack of widespread awareness regarding the early signs and management of respiratory diseases can delay diagnosis and treatment, impacting market demand for diagnostic devices.
Emerging Opportunities in Brazil Respiratory Devices Industry
Emerging opportunities in the Brazil respiratory devices industry are centered around the burgeoning home healthcare market and the integration of digital health solutions. The increasing demand for remote patient monitoring and telehealth services presents a significant avenue for growth for connected respiratory devices, such as smart CPAP machines and portable ventilators. Furthermore, the untapped potential in rural and underserved regions of Brazil offers a substantial market for more affordable and accessible diagnostic and therapeutic respiratory solutions. The rising adoption of personalized medicine approaches also opens doors for innovative applications of respiratory devices that can tailor treatment based on individual patient data and genetic predispositions.
Growth Accelerators in the Brazil Respiratory Devices Industry Industry
Several catalysts are propelling long-term growth in the Brazil respiratory devices industry. Technological breakthroughs, particularly in areas like AI-driven diagnostics for early disease detection and personalized treatment algorithms, are enhancing the efficacy and appeal of respiratory care. Strategic partnerships between device manufacturers, healthcare providers, and technology companies are fostering innovation and expanding market reach. Market expansion strategies, including the development of more affordable product lines and targeted distribution networks for rural areas, are crucial for capturing a larger share of the Brazilian market. The increasing focus on preventative healthcare and the management of chronic diseases by both government and private entities further solidifies the positive growth trajectory for the industry.
Key Players Shaping the Brazil Respiratory Devices Industry Market
- Hamilton Medical
- Drägerwerk AG & Co KGaA
- BD (Becton Dickinson and Company)
- Masimo
- Medtronic
- Fisher & Paykel Healthcare Limited
- Koninklijke Philips NV
- ResMed Inc
- Chart Industries
- General Electric Company (GE HealthCare)
Notable Milestones in Brazil Respiratory Devices Industry Sector
- April 2022: ANVISA, Brazil's medical device market regulator, issued a new resolution updating Brazilian Good Manufacturing Practices (BGMP) for medical devices. ANVISA's RDC 665/2022 replaced previous regulations, including RDC 16/2013 and IN 08/2013.
- April 2022: Global oxygen equipment manufacturer CAIRE Inc. is expanding its portfolio of solutions in Latin America by launching Companion 5 and NewLife Intensity 10 stationary oxygen concentrators in Brazil following an anticipated approval by the Brazilian Health Regularity Agency (Anvisa).
In-Depth Brazil Respiratory Devices Industry Market Outlook
The Brazil respiratory devices industry is characterized by a promising future, driven by sustained demand for essential respiratory care solutions. Growth accelerators, including continuous technological innovation in connected devices and AI-powered diagnostics, will enable more personalized and accessible patient care. Strategic collaborations and market expansion initiatives targeting underserved populations and home healthcare settings are expected to further unlock market potential. The industry's outlook is further bolstered by increasing government focus on public health and chronic disease management, creating a favorable environment for sustained growth and investment. By leveraging these opportunities, stakeholders can effectively navigate the evolving landscape and capitalize on the significant expansion potential within Brazil's dynamic respiratory devices market.
Brazil Respiratory Devices Industry Segmentation
-
1. Diagnostic and Monitoring Devices
- 1.1. Spirometers
- 1.2. Sleep Test Devices
- 1.3. Peak Flow Meters
- 1.4. Pulse Oximeters
- 1.5. Capnographs
- 1.6. Other Diagnostic and Monitoring Devices
-
2. Therapeutic Devices
- 2.1. CPAP Devices
- 2.2. BiPAP Devices
- 2.3. Humidifiers
- 2.4. Nebulizers
- 2.5. Oxygen Concentrators
- 2.6. Ventilators
- 2.7. Inhalers
- 2.8. Other Therapeutic Devices
-
3. Disposables
- 3.1. Masks
- 3.2. Breathing Circuit
- 3.3. Other Disposables
Brazil Respiratory Devices Industry Segmentation By Geography
- 1. Brazil
Brazil Respiratory Devices Industry Regional Market Share

Geographic Coverage of Brazil Respiratory Devices Industry
Brazil Respiratory Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.5% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Respiratory Disorders Such as COPD and TB; Technological Advancements and Increasing Applications in Homecare Setting
- 3.3. Market Restrains
- 3.3.1. High Cost of Devices
- 3.4. Market Trends
- 3.4.1. Inhalers Expected to Grow Fastest During the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Brazil Respiratory Devices Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Diagnostic and Monitoring Devices
- 5.1.1. Spirometers
- 5.1.2. Sleep Test Devices
- 5.1.3. Peak Flow Meters
- 5.1.4. Pulse Oximeters
- 5.1.5. Capnographs
- 5.1.6. Other Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Therapeutic Devices
- 5.2.1. CPAP Devices
- 5.2.2. BiPAP Devices
- 5.2.3. Humidifiers
- 5.2.4. Nebulizers
- 5.2.5. Oxygen Concentrators
- 5.2.6. Ventilators
- 5.2.7. Inhalers
- 5.2.8. Other Therapeutic Devices
- 5.3. Market Analysis, Insights and Forecast - by Disposables
- 5.3.1. Masks
- 5.3.2. Breathing Circuit
- 5.3.3. Other Disposables
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. Brazil
- 5.1. Market Analysis, Insights and Forecast - by Diagnostic and Monitoring Devices
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Hamilton Medical
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Drägerwerk AG & Co KGaA
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 BD (Becton Dickinson and Company)*List Not Exhaustive
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Masimo
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Medtronic
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Fisher & Paykel Healthcare Limited
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Koninklijke Philips NV
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 ResMed Inc
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Chart Industries
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 General Electric Company (GE HealthCare)
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.1 Hamilton Medical
List of Figures
- Figure 1: Brazil Respiratory Devices Industry Revenue Breakdown (million, %) by Product 2025 & 2033
- Figure 2: Brazil Respiratory Devices Industry Share (%) by Company 2025
List of Tables
- Table 1: Brazil Respiratory Devices Industry Revenue million Forecast, by Diagnostic and Monitoring Devices 2020 & 2033
- Table 2: Brazil Respiratory Devices Industry Volume K Units Forecast, by Diagnostic and Monitoring Devices 2020 & 2033
- Table 3: Brazil Respiratory Devices Industry Revenue million Forecast, by Therapeutic Devices 2020 & 2033
- Table 4: Brazil Respiratory Devices Industry Volume K Units Forecast, by Therapeutic Devices 2020 & 2033
- Table 5: Brazil Respiratory Devices Industry Revenue million Forecast, by Disposables 2020 & 2033
- Table 6: Brazil Respiratory Devices Industry Volume K Units Forecast, by Disposables 2020 & 2033
- Table 7: Brazil Respiratory Devices Industry Revenue million Forecast, by Region 2020 & 2033
- Table 8: Brazil Respiratory Devices Industry Volume K Units Forecast, by Region 2020 & 2033
- Table 9: Brazil Respiratory Devices Industry Revenue million Forecast, by Diagnostic and Monitoring Devices 2020 & 2033
- Table 10: Brazil Respiratory Devices Industry Volume K Units Forecast, by Diagnostic and Monitoring Devices 2020 & 2033
- Table 11: Brazil Respiratory Devices Industry Revenue million Forecast, by Therapeutic Devices 2020 & 2033
- Table 12: Brazil Respiratory Devices Industry Volume K Units Forecast, by Therapeutic Devices 2020 & 2033
- Table 13: Brazil Respiratory Devices Industry Revenue million Forecast, by Disposables 2020 & 2033
- Table 14: Brazil Respiratory Devices Industry Volume K Units Forecast, by Disposables 2020 & 2033
- Table 15: Brazil Respiratory Devices Industry Revenue million Forecast, by Country 2020 & 2033
- Table 16: Brazil Respiratory Devices Industry Volume K Units Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Brazil Respiratory Devices Industry?
The projected CAGR is approximately 6.5%.
2. Which companies are prominent players in the Brazil Respiratory Devices Industry?
Key companies in the market include Hamilton Medical, Drägerwerk AG & Co KGaA, BD (Becton Dickinson and Company)*List Not Exhaustive, Masimo, Medtronic, Fisher & Paykel Healthcare Limited, Koninklijke Philips NV, ResMed Inc, Chart Industries, General Electric Company (GE HealthCare).
3. What are the main segments of the Brazil Respiratory Devices Industry?
The market segments include Diagnostic and Monitoring Devices, Therapeutic Devices, Disposables.
4. Can you provide details about the market size?
The market size is estimated to be USD 343.74 million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Respiratory Disorders Such as COPD and TB; Technological Advancements and Increasing Applications in Homecare Setting.
6. What are the notable trends driving market growth?
Inhalers Expected to Grow Fastest During the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Devices.
8. Can you provide examples of recent developments in the market?
April 2022: ANVISA, Brazil's medical device market regulator, issued a new resolution updating Brazilian Good Manufacturing Practices (BGMP) for medical devices. ANVISA's RDC 665/2022 replaced previous regulations, including RDC 16/2013 and IN 08/2013.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Brazil Respiratory Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Brazil Respiratory Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Brazil Respiratory Devices Industry?
To stay informed about further developments, trends, and reports in the Brazil Respiratory Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
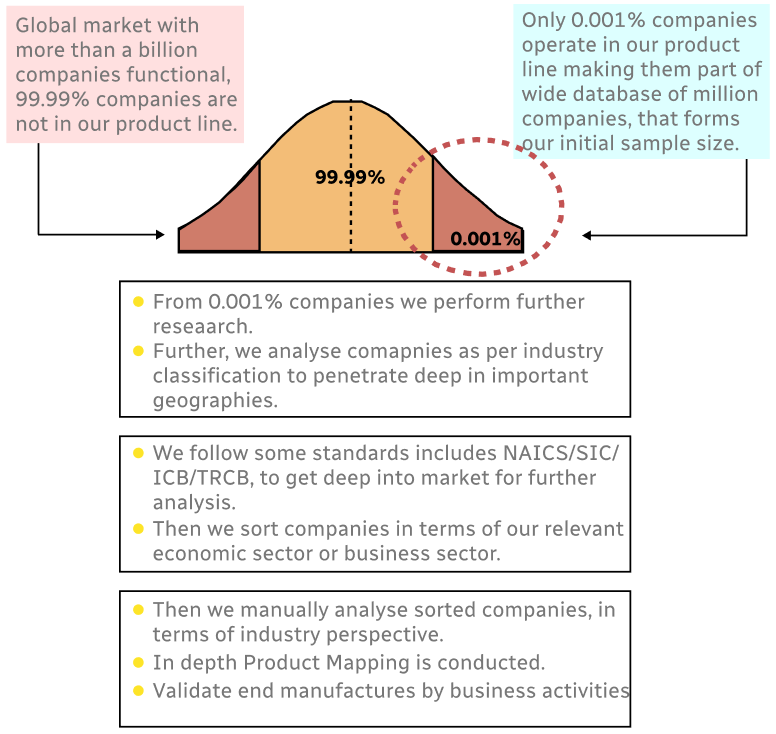
Step 1 - Identification of Relevant Samples Size from Population Database
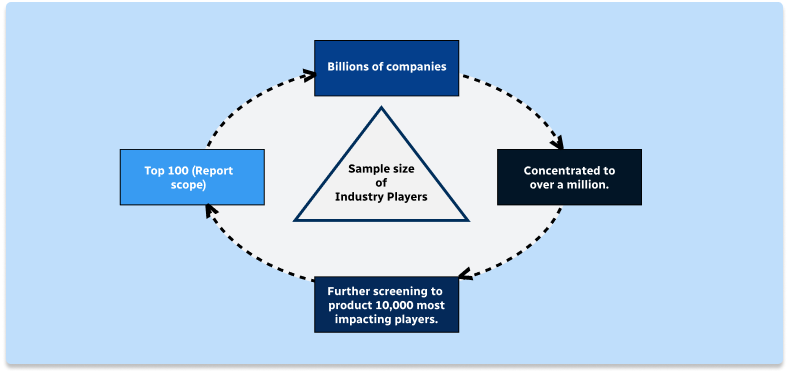
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
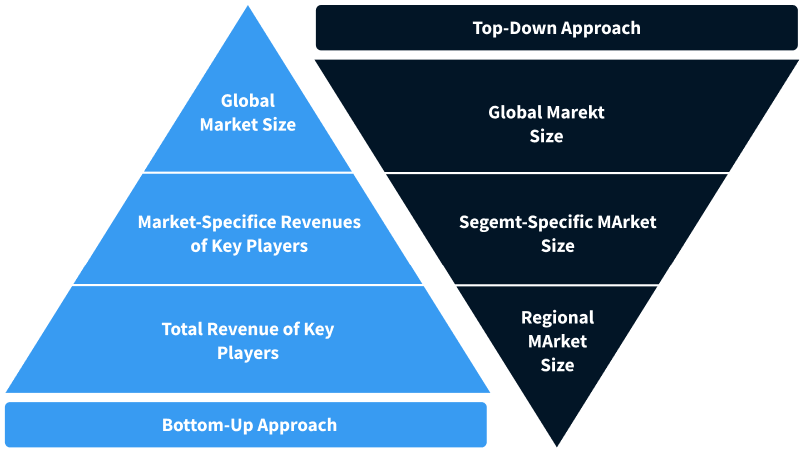
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


