Key Insights
The global Cytology market is poised for substantial expansion, projected to reach \$20.54 million by 2025 and exhibit a robust Compound Annual Growth Rate (CAGR) of 13.50% through 2033. This significant growth is fueled by an increasing global burden of diseases requiring cytological examination, particularly cancers such as breast and cervical cancer. Advances in diagnostic technologies, including enhanced microscopy techniques like Fluorescent In-situ Hybridization (FISH), the growing adoption of Polymerase Chain Reaction (PCR) for genetic analysis, and the increasing utilization of molecular genetics tests, are key drivers. Furthermore, the expanding role of flow cytometry in disease detection and monitoring is contributing to market momentum. The growing awareness among healthcare providers and patients about the benefits of early disease detection through cytology also plays a crucial role.
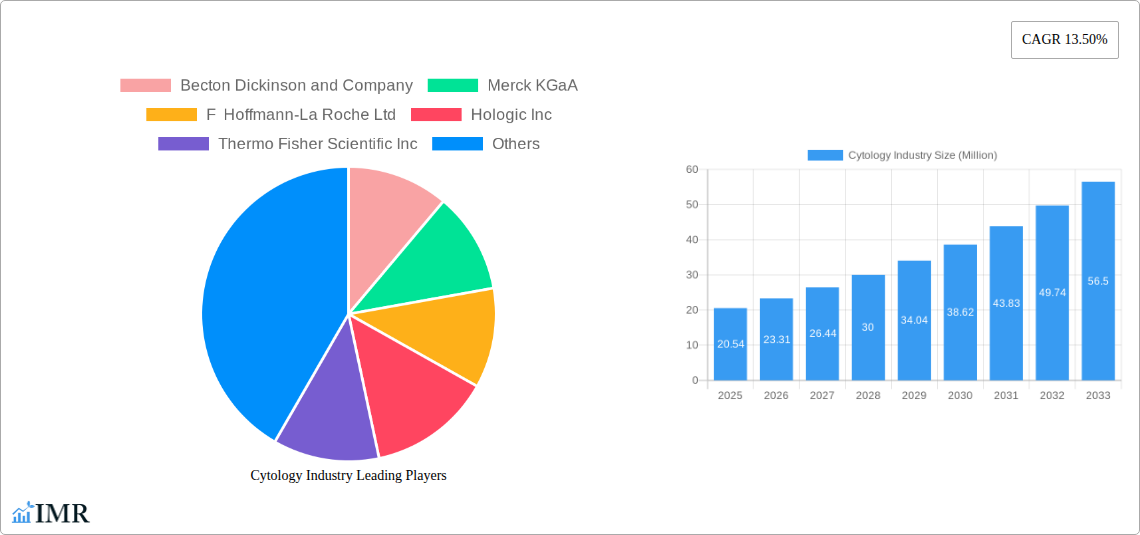
Cytology Industry Market Size (In Million)

The market's trajectory is further shaped by several prevailing trends. The rising demand for minimally invasive diagnostic procedures, coupled with technological innovations leading to more accurate and faster results, is accelerating adoption. Hospitals and clinics are increasingly investing in advanced cytology equipment to improve diagnostic capabilities, while academic and research institutes are driving innovation and expanding the scope of cytological applications. Geographically, North America and Europe currently lead the market, driven by high healthcare expenditure and advanced infrastructure. However, the Asia Pacific region is anticipated to witness the fastest growth due to its large population, rising healthcare awareness, and increasing investments in diagnostic facilities. While the market demonstrates a strong upward trend, potential restraints could include the high cost of advanced equipment and the need for skilled professionals to operate them.
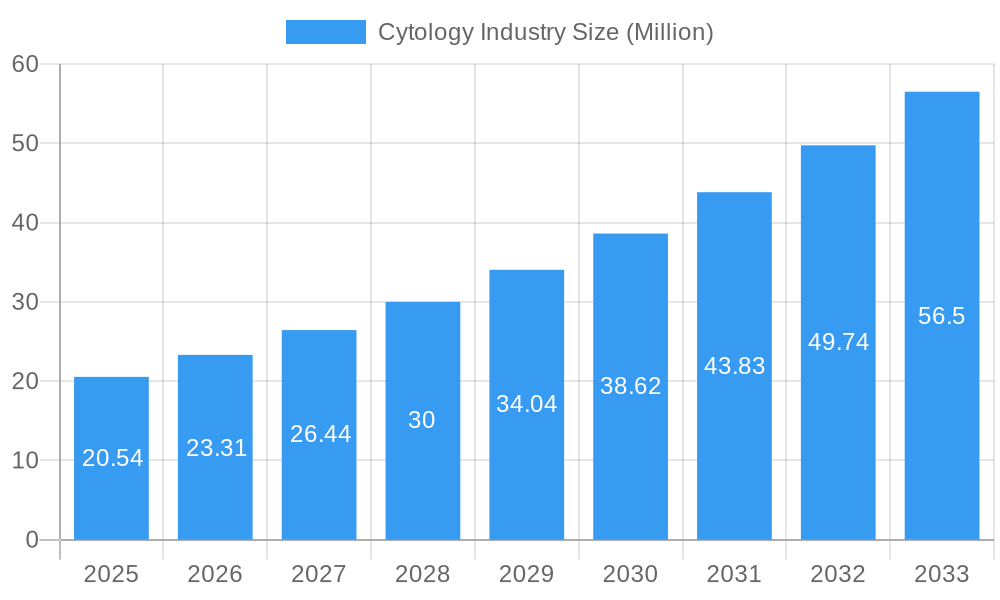
Cytology Industry Company Market Share

Here's a comprehensive, SEO-optimized report description for the Cytology Industry, integrating high-traffic keywords and structured for maximum impact:
Cytology Industry Market Dynamics & Structure
The global cytology market is characterized by a moderately consolidated structure, with key players like Becton Dickinson and Company, Merck KGaA, and F Hoffmann-La Roche Ltd holding significant market shares. Technological innovation is a primary driver, fueled by advancements in molecular diagnostics and automated imaging systems. Regulatory frameworks, particularly stringent FDA approvals for diagnostic tests, influence product development and market entry. Competitive product substitutes include more invasive diagnostic procedures, but the minimally invasive nature of cytology continues to offer a strong value proposition. End-user demographics are shifting towards an aging population and increasing awareness of early disease detection, especially for prevalent cancers like breast and cervical cancer. Mergers and acquisitions (M&A) are a notable trend, with companies strategically acquiring smaller innovators to expand their portfolios and market reach, contributing to market consolidation. For instance, in the historical period (2019-2024), an estimated XX M&A deals were recorded, averaging $XX million in value, indicating strategic consolidation and expansion. Barriers to innovation include the high cost of research and development and the lengthy regulatory approval processes.
- Market Concentration: Moderately consolidated with leading global players.
- Technological Innovation Drivers: Molecular diagnostics, automation, AI in image analysis.
- Regulatory Frameworks: Strict FDA and EMA approvals for diagnostic kits and devices.
- Competitive Substitutes: Biopsies, advanced imaging techniques (MRI, CT).
- End-User Demographics: Aging populations, increased cancer screening prevalence.
- M&A Trends: Strategic acquisitions for portfolio expansion and market access.
Cytology Industry Growth Trends & Insights
The global cytology market is poised for robust growth, projected to expand from an estimated $XXXX million in 2025 to $XXXX million by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of XX% during the forecast period (2025–2033). This expansion is underpinned by several critical trends. The increasing incidence of cancers, particularly breast cancer and cervical cancer, is a significant market driver. Advances in diagnostic technologies, such as the integration of artificial intelligence (AI) for automated slide analysis and the development of more sensitive molecular tests for HPV and other biomarkers, are enhancing diagnostic accuracy and efficiency, leading to higher adoption rates. The growing emphasis on early disease detection and preventative healthcare globally is further propelling market growth. Improved diagnostic platforms and higher throughput automation in laboratories are contributing to increased testing volumes. Furthermore, the rising demand for personalized medicine and targeted therapies necessitates more precise diagnostic tools, a niche that cytology is increasingly fulfilling. Consumer awareness regarding the importance of regular screening for early detection of various cancers is also on the rise, contributing to increased demand for cytology-based diagnostics. The market penetration of advanced cytology techniques, like liquid-based cytology (LBC) and molecular testing, is expected to surge as healthcare providers and patients recognize their superior diagnostic capabilities compared to conventional methods. Disruptions like the February 2023 announcement by BioReference Health LLC to offer Roche Diagnostics' CINtec PLUS Cytology test, an FDA-approved dual-stain triage test for HPV-positive patients, exemplify the rapid innovation and adoption of new technologies aimed at improving patient outcomes and streamlining diagnostic pathways.
Dominant Regions, Countries, or Segments in Cytology Industry
North America currently dominates the global cytology market, driven by its well-established healthcare infrastructure, high per capita healthcare expenditure, and a strong emphasis on advanced diagnostic technologies. The United States, in particular, is a leading contributor, fueled by widespread adoption of molecular diagnostic tests, extensive cancer screening programs, and significant investment in research and development by key industry players. The prevalence of breast cancer and cervical cancer, coupled with proactive government initiatives for early detection, further solidifies North America's leading position.
Within the Type of Examination, Cytology itself, encompassing breast cancer and cervical cancer screening, represents the largest segment. The widespread implementation of Pap smears and HPV testing for cervical cancer, along with advancements in mammography and liquid biopsy techniques for breast cancer, significantly contribute to this segment's dominance. The Test Type segment of Molecular Genetics Tests is exhibiting the fastest growth, driven by the increasing demand for precise genetic analysis in cancer diagnosis and personalized treatment.
The End User segment of Hospitals and Clinics accounts for the largest market share due to their direct patient interaction and the volume of diagnostic tests performed. However, Academic and Research Institutes are crucial drivers of innovation and the adoption of novel technologies.
Key drivers for regional dominance include:
- Economic Policies: Favorable reimbursement policies for diagnostic procedures and government funding for healthcare research.
- Infrastructure: Advanced laboratory facilities, sophisticated diagnostic equipment, and a skilled workforce.
- Technological Adoption: Early and rapid uptake of new diagnostic technologies and automated systems.
- Disease Prevalence: High incidence rates of target cancers like breast and cervical cancer.
- Regulatory Support: Streamlined regulatory pathways for innovative diagnostic solutions.
The market share of North America is estimated at XX% of the global cytology market in 2025, with an anticipated CAGR of XX% during the forecast period. The growth potential in emerging economies within Asia-Pacific and Latin America presents significant opportunities for future market expansion.
Cytology Industry Product Landscape
The cytology industry is marked by continuous product innovation, with a focus on enhancing diagnostic accuracy, improving workflow efficiency, and expanding the scope of detectable biomarkers. Key product categories include automated slide stainers, digital imaging systems, liquid-based cytology preparations, and advanced molecular diagnostic kits for HPV, genetic mutations, and other cancer-related markers. These products offer significant performance improvements, such as reduced turnaround times, increased sensitivity and specificity, and the ability to perform multiplexed testing from a single sample. Unique selling propositions often revolve around AI-powered image analysis for earlier and more accurate detection, integration with laboratory information systems (LIS), and the development of non-invasive or minimally invasive diagnostic solutions like liquid biopsies for various cancers, including bladder cancer as exemplified by Nanostics Inc.'s Clarity DX Bladder platform.
Key Drivers, Barriers & Challenges in Cytology Industry
Key Drivers:
- Rising Cancer Incidence: Increasing global prevalence of cancer, particularly breast and cervical cancer, fuels demand for diagnostic testing.
- Technological Advancements: Development of automated systems, AI-driven image analysis, and sensitive molecular diagnostics enhances accuracy and efficiency.
- Preventative Healthcare Focus: Growing emphasis on early disease detection and screening programs worldwide.
- Government Initiatives: Supportive policies and funding for cancer research and public health initiatives.
Key Barriers & Challenges:
- High Cost of Equipment & Reagents: Significant capital investment and recurring operational costs for advanced cytology laboratories.
- Regulatory Hurdles: Stringent and time-consuming approval processes for new diagnostic tests and devices can delay market entry.
- Skilled Workforce Shortage: Demand for highly trained cytotechnologists and pathologists can outpace supply.
- Reimbursement Policies: Inconsistent or inadequate reimbursement for certain cytology tests can impact adoption rates.
- Competition: Intense competition from established players and emerging innovators.
- Supply Chain Disruptions: Potential for disruptions in the availability of critical reagents and consumables, impacting laboratory operations. The impact of such disruptions can lead to delays in testing, affecting patient care and potentially costing laboratories millions in lost revenue and increased operational expenses.
Emerging Opportunities in Cytology Industry
Emerging opportunities in the cytology industry are primarily centered on the expansion of liquid biopsy technologies for non-invasive cancer detection and monitoring, particularly for challenging-to-access cancers. The integration of artificial intelligence (AI) and machine learning (ML) into digital pathology workflows presents a significant opportunity to improve diagnostic accuracy, reduce pathologist workload, and accelerate turnaround times. Furthermore, the increasing demand for personalized medicine is driving the development of multi-analyte tests that can simultaneously detect various biomarkers from a single sample, enabling more targeted therapeutic decisions. Untapped markets in developing regions, with growing healthcare expenditure and increasing awareness of cancer screening, also represent a substantial growth avenue. The development of point-of-care diagnostic solutions for cytology, enabling faster results in remote or underserved areas, is another promising area.
Growth Accelerators in the Cytology Industry Industry
Long-term growth in the cytology industry will be significantly accelerated by breakthroughs in nanotechnology for highly sensitive biomarker detection, enabling earlier and more accurate diagnoses. Strategic partnerships between diagnostic companies, pharmaceutical firms, and academic institutions will foster collaborative research and development, leading to the creation of novel diagnostic-therapeutic combinations. Market expansion strategies targeting underserved populations and emerging economies, coupled with increased investment in automation and digital pathology infrastructure, will also be crucial growth catalysts. The ongoing evolution of companion diagnostics, linked to targeted cancer therapies, will further solidify the importance of advanced cytology techniques in precision medicine.
Key Players Shaping the Cytology Industry Market
- Becton Dickinson and Company
- Merck KGaA
- F Hoffmann-La Roche Ltd
- Hologic Inc
- Thermo Fisher Scientific Inc
- Trivitron Healthcare
- Danaher Corporation
- Abbott
- PerkinElmer Inc
- Sysmex Corporation
Notable Milestones in Cytology Industry Sector
- February 2023: BioReference Health LLC announced it would be one of the first commercial laboratories to offer the CINtec PLUS Cytology test from Roche Diagnostics. CINtec PLUS Cytology is the only FDA-approved dual-stain triage test for patients who have a positive high-risk human papillomavirus (HPV) result. This development enhances cervical cancer screening protocols.
- July 2022: Nanostics Inc. launched a prospective clinical study to validate a novel and minimally invasive bladder cancer diagnostic test, Clarity DX Bladder, using its Clarity DX diagnostic platform. This milestone signifies progress in non-invasive cancer diagnostics.
In-Depth Cytology Industry Market Outlook
The future outlook for the cytology industry is exceptionally bright, propelled by an unwavering commitment to advancing diagnostic capabilities and improving patient outcomes. Growth accelerators such as AI-driven pathology, the burgeoning field of liquid biopsies, and the increasing demand for personalized medicine will continue to shape the market landscape. Strategic collaborations and investments in emerging markets will unlock new revenue streams and expand access to critical diagnostic services. The industry is poised to witness transformative innovations that will redefine cancer screening and diagnosis, making earlier detection and more effective treatments a reality for a broader patient population. The projected market size of $XXXX million by 2033 underscores the sustained demand and growth potential within this vital sector.
Cytology Industry Segmentation
-
1. Type of Examination
- 1.1. Histology
-
1.2. Cytology
- 1.2.1. Breast Cancer
- 1.2.2. Cervical Cancer
- 1.2.3. Others
-
2. Test Type
-
2.1. Microscopy Tests
-
2.1.1. Cytogenic Tests
- 2.1.1.1. Karyotyping
- 2.1.1.2. Fluorescent In-situ Hybridization (FISH)
- 2.1.2. Polymerase Chain Reaction
- 2.1.3. Other Microscopy Tests
-
2.1.1. Cytogenic Tests
- 2.2. Molecular Genetics Tests
- 2.3. Flow Cytomtery
-
2.1. Microscopy Tests
-
3. End User
- 3.1. Hospitals and Clinics
- 3.2. Academic and Research Institutes
- 3.3. Other End Users
Cytology Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
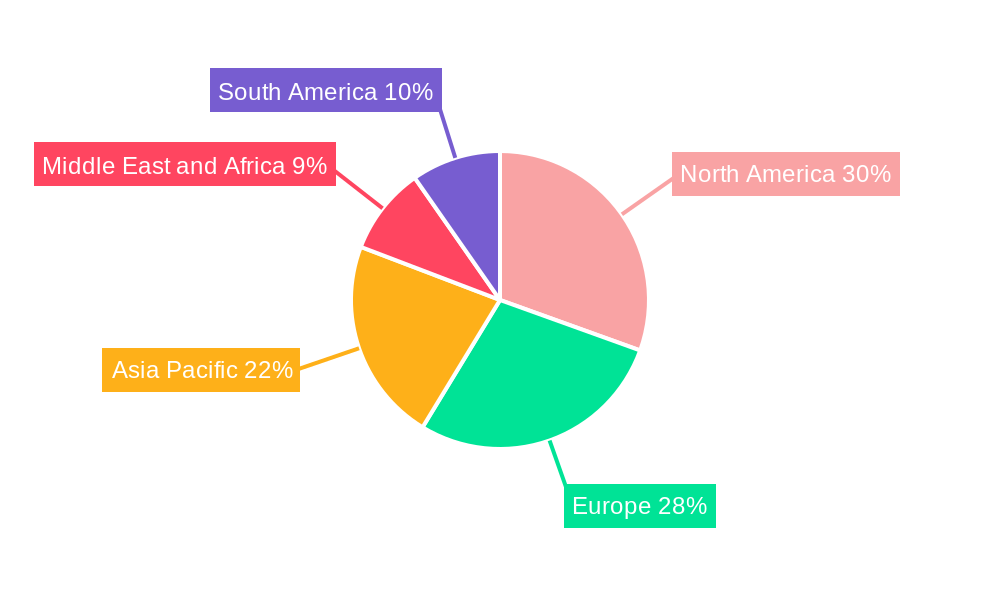
Cytology Industry Regional Market Share

Geographic Coverage of Cytology Industry
Cytology Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 13.50% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Prevalence of Cancer; Increasing Standardization of Pathological Laboratories; Technological Advancements in Diagnostic and Molecular Techniques
- 3.3. Market Restrains
- 3.3.1. Safety Issues and Diagnostic Accuracy Issues with Histopathological and Cytopathological Tests; Lack of Awareness among the Public for Diagnostic Tests
- 3.4. Market Trends
- 3.4.1. Cervical Cancer Segment is Expected to Have a Significant Market Share During the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Cytology Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Type of Examination
- 5.1.1. Histology
- 5.1.2. Cytology
- 5.1.2.1. Breast Cancer
- 5.1.2.2. Cervical Cancer
- 5.1.2.3. Others
- 5.2. Market Analysis, Insights and Forecast - by Test Type
- 5.2.1. Microscopy Tests
- 5.2.1.1. Cytogenic Tests
- 5.2.1.1.1. Karyotyping
- 5.2.1.1.2. Fluorescent In-situ Hybridization (FISH)
- 5.2.1.2. Polymerase Chain Reaction
- 5.2.1.3. Other Microscopy Tests
- 5.2.1.1. Cytogenic Tests
- 5.2.2. Molecular Genetics Tests
- 5.2.3. Flow Cytomtery
- 5.2.1. Microscopy Tests
- 5.3. Market Analysis, Insights and Forecast - by End User
- 5.3.1. Hospitals and Clinics
- 5.3.2. Academic and Research Institutes
- 5.3.3. Other End Users
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Middle East and Africa
- 5.4.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Type of Examination
- 6. North America Cytology Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Type of Examination
- 6.1.1. Histology
- 6.1.2. Cytology
- 6.1.2.1. Breast Cancer
- 6.1.2.2. Cervical Cancer
- 6.1.2.3. Others
- 6.2. Market Analysis, Insights and Forecast - by Test Type
- 6.2.1. Microscopy Tests
- 6.2.1.1. Cytogenic Tests
- 6.2.1.1.1. Karyotyping
- 6.2.1.1.2. Fluorescent In-situ Hybridization (FISH)
- 6.2.1.2. Polymerase Chain Reaction
- 6.2.1.3. Other Microscopy Tests
- 6.2.1.1. Cytogenic Tests
- 6.2.2. Molecular Genetics Tests
- 6.2.3. Flow Cytomtery
- 6.2.1. Microscopy Tests
- 6.3. Market Analysis, Insights and Forecast - by End User
- 6.3.1. Hospitals and Clinics
- 6.3.2. Academic and Research Institutes
- 6.3.3. Other End Users
- 6.1. Market Analysis, Insights and Forecast - by Type of Examination
- 7. Europe Cytology Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Type of Examination
- 7.1.1. Histology
- 7.1.2. Cytology
- 7.1.2.1. Breast Cancer
- 7.1.2.2. Cervical Cancer
- 7.1.2.3. Others
- 7.2. Market Analysis, Insights and Forecast - by Test Type
- 7.2.1. Microscopy Tests
- 7.2.1.1. Cytogenic Tests
- 7.2.1.1.1. Karyotyping
- 7.2.1.1.2. Fluorescent In-situ Hybridization (FISH)
- 7.2.1.2. Polymerase Chain Reaction
- 7.2.1.3. Other Microscopy Tests
- 7.2.1.1. Cytogenic Tests
- 7.2.2. Molecular Genetics Tests
- 7.2.3. Flow Cytomtery
- 7.2.1. Microscopy Tests
- 7.3. Market Analysis, Insights and Forecast - by End User
- 7.3.1. Hospitals and Clinics
- 7.3.2. Academic and Research Institutes
- 7.3.3. Other End Users
- 7.1. Market Analysis, Insights and Forecast - by Type of Examination
- 8. Asia Pacific Cytology Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Type of Examination
- 8.1.1. Histology
- 8.1.2. Cytology
- 8.1.2.1. Breast Cancer
- 8.1.2.2. Cervical Cancer
- 8.1.2.3. Others
- 8.2. Market Analysis, Insights and Forecast - by Test Type
- 8.2.1. Microscopy Tests
- 8.2.1.1. Cytogenic Tests
- 8.2.1.1.1. Karyotyping
- 8.2.1.1.2. Fluorescent In-situ Hybridization (FISH)
- 8.2.1.2. Polymerase Chain Reaction
- 8.2.1.3. Other Microscopy Tests
- 8.2.1.1. Cytogenic Tests
- 8.2.2. Molecular Genetics Tests
- 8.2.3. Flow Cytomtery
- 8.2.1. Microscopy Tests
- 8.3. Market Analysis, Insights and Forecast - by End User
- 8.3.1. Hospitals and Clinics
- 8.3.2. Academic and Research Institutes
- 8.3.3. Other End Users
- 8.1. Market Analysis, Insights and Forecast - by Type of Examination
- 9. Middle East and Africa Cytology Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Type of Examination
- 9.1.1. Histology
- 9.1.2. Cytology
- 9.1.2.1. Breast Cancer
- 9.1.2.2. Cervical Cancer
- 9.1.2.3. Others
- 9.2. Market Analysis, Insights and Forecast - by Test Type
- 9.2.1. Microscopy Tests
- 9.2.1.1. Cytogenic Tests
- 9.2.1.1.1. Karyotyping
- 9.2.1.1.2. Fluorescent In-situ Hybridization (FISH)
- 9.2.1.2. Polymerase Chain Reaction
- 9.2.1.3. Other Microscopy Tests
- 9.2.1.1. Cytogenic Tests
- 9.2.2. Molecular Genetics Tests
- 9.2.3. Flow Cytomtery
- 9.2.1. Microscopy Tests
- 9.3. Market Analysis, Insights and Forecast - by End User
- 9.3.1. Hospitals and Clinics
- 9.3.2. Academic and Research Institutes
- 9.3.3. Other End Users
- 9.1. Market Analysis, Insights and Forecast - by Type of Examination
- 10. South America Cytology Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Type of Examination
- 10.1.1. Histology
- 10.1.2. Cytology
- 10.1.2.1. Breast Cancer
- 10.1.2.2. Cervical Cancer
- 10.1.2.3. Others
- 10.2. Market Analysis, Insights and Forecast - by Test Type
- 10.2.1. Microscopy Tests
- 10.2.1.1. Cytogenic Tests
- 10.2.1.1.1. Karyotyping
- 10.2.1.1.2. Fluorescent In-situ Hybridization (FISH)
- 10.2.1.2. Polymerase Chain Reaction
- 10.2.1.3. Other Microscopy Tests
- 10.2.1.1. Cytogenic Tests
- 10.2.2. Molecular Genetics Tests
- 10.2.3. Flow Cytomtery
- 10.2.1. Microscopy Tests
- 10.3. Market Analysis, Insights and Forecast - by End User
- 10.3.1. Hospitals and Clinics
- 10.3.2. Academic and Research Institutes
- 10.3.3. Other End Users
- 10.1. Market Analysis, Insights and Forecast - by Type of Examination
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Becton Dickinson and Company
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Merck KGaA
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 F Hoffmann-La Roche Ltd
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Hologic Inc
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Thermo Fisher Scientific Inc
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Trivitron Healthcare
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Danaher Corporation
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Abbott
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 PerkinElmer Inc
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Sysmex Corporation
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.1 Becton Dickinson and Company
List of Figures
- Figure 1: Global Cytology Industry Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: Global Cytology Industry Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: North America Cytology Industry Revenue (Million), by Type of Examination 2025 & 2033
- Figure 4: North America Cytology Industry Volume (K Unit), by Type of Examination 2025 & 2033
- Figure 5: North America Cytology Industry Revenue Share (%), by Type of Examination 2025 & 2033
- Figure 6: North America Cytology Industry Volume Share (%), by Type of Examination 2025 & 2033
- Figure 7: North America Cytology Industry Revenue (Million), by Test Type 2025 & 2033
- Figure 8: North America Cytology Industry Volume (K Unit), by Test Type 2025 & 2033
- Figure 9: North America Cytology Industry Revenue Share (%), by Test Type 2025 & 2033
- Figure 10: North America Cytology Industry Volume Share (%), by Test Type 2025 & 2033
- Figure 11: North America Cytology Industry Revenue (Million), by End User 2025 & 2033
- Figure 12: North America Cytology Industry Volume (K Unit), by End User 2025 & 2033
- Figure 13: North America Cytology Industry Revenue Share (%), by End User 2025 & 2033
- Figure 14: North America Cytology Industry Volume Share (%), by End User 2025 & 2033
- Figure 15: North America Cytology Industry Revenue (Million), by Country 2025 & 2033
- Figure 16: North America Cytology Industry Volume (K Unit), by Country 2025 & 2033
- Figure 17: North America Cytology Industry Revenue Share (%), by Country 2025 & 2033
- Figure 18: North America Cytology Industry Volume Share (%), by Country 2025 & 2033
- Figure 19: Europe Cytology Industry Revenue (Million), by Type of Examination 2025 & 2033
- Figure 20: Europe Cytology Industry Volume (K Unit), by Type of Examination 2025 & 2033
- Figure 21: Europe Cytology Industry Revenue Share (%), by Type of Examination 2025 & 2033
- Figure 22: Europe Cytology Industry Volume Share (%), by Type of Examination 2025 & 2033
- Figure 23: Europe Cytology Industry Revenue (Million), by Test Type 2025 & 2033
- Figure 24: Europe Cytology Industry Volume (K Unit), by Test Type 2025 & 2033
- Figure 25: Europe Cytology Industry Revenue Share (%), by Test Type 2025 & 2033
- Figure 26: Europe Cytology Industry Volume Share (%), by Test Type 2025 & 2033
- Figure 27: Europe Cytology Industry Revenue (Million), by End User 2025 & 2033
- Figure 28: Europe Cytology Industry Volume (K Unit), by End User 2025 & 2033
- Figure 29: Europe Cytology Industry Revenue Share (%), by End User 2025 & 2033
- Figure 30: Europe Cytology Industry Volume Share (%), by End User 2025 & 2033
- Figure 31: Europe Cytology Industry Revenue (Million), by Country 2025 & 2033
- Figure 32: Europe Cytology Industry Volume (K Unit), by Country 2025 & 2033
- Figure 33: Europe Cytology Industry Revenue Share (%), by Country 2025 & 2033
- Figure 34: Europe Cytology Industry Volume Share (%), by Country 2025 & 2033
- Figure 35: Asia Pacific Cytology Industry Revenue (Million), by Type of Examination 2025 & 2033
- Figure 36: Asia Pacific Cytology Industry Volume (K Unit), by Type of Examination 2025 & 2033
- Figure 37: Asia Pacific Cytology Industry Revenue Share (%), by Type of Examination 2025 & 2033
- Figure 38: Asia Pacific Cytology Industry Volume Share (%), by Type of Examination 2025 & 2033
- Figure 39: Asia Pacific Cytology Industry Revenue (Million), by Test Type 2025 & 2033
- Figure 40: Asia Pacific Cytology Industry Volume (K Unit), by Test Type 2025 & 2033
- Figure 41: Asia Pacific Cytology Industry Revenue Share (%), by Test Type 2025 & 2033
- Figure 42: Asia Pacific Cytology Industry Volume Share (%), by Test Type 2025 & 2033
- Figure 43: Asia Pacific Cytology Industry Revenue (Million), by End User 2025 & 2033
- Figure 44: Asia Pacific Cytology Industry Volume (K Unit), by End User 2025 & 2033
- Figure 45: Asia Pacific Cytology Industry Revenue Share (%), by End User 2025 & 2033
- Figure 46: Asia Pacific Cytology Industry Volume Share (%), by End User 2025 & 2033
- Figure 47: Asia Pacific Cytology Industry Revenue (Million), by Country 2025 & 2033
- Figure 48: Asia Pacific Cytology Industry Volume (K Unit), by Country 2025 & 2033
- Figure 49: Asia Pacific Cytology Industry Revenue Share (%), by Country 2025 & 2033
- Figure 50: Asia Pacific Cytology Industry Volume Share (%), by Country 2025 & 2033
- Figure 51: Middle East and Africa Cytology Industry Revenue (Million), by Type of Examination 2025 & 2033
- Figure 52: Middle East and Africa Cytology Industry Volume (K Unit), by Type of Examination 2025 & 2033
- Figure 53: Middle East and Africa Cytology Industry Revenue Share (%), by Type of Examination 2025 & 2033
- Figure 54: Middle East and Africa Cytology Industry Volume Share (%), by Type of Examination 2025 & 2033
- Figure 55: Middle East and Africa Cytology Industry Revenue (Million), by Test Type 2025 & 2033
- Figure 56: Middle East and Africa Cytology Industry Volume (K Unit), by Test Type 2025 & 2033
- Figure 57: Middle East and Africa Cytology Industry Revenue Share (%), by Test Type 2025 & 2033
- Figure 58: Middle East and Africa Cytology Industry Volume Share (%), by Test Type 2025 & 2033
- Figure 59: Middle East and Africa Cytology Industry Revenue (Million), by End User 2025 & 2033
- Figure 60: Middle East and Africa Cytology Industry Volume (K Unit), by End User 2025 & 2033
- Figure 61: Middle East and Africa Cytology Industry Revenue Share (%), by End User 2025 & 2033
- Figure 62: Middle East and Africa Cytology Industry Volume Share (%), by End User 2025 & 2033
- Figure 63: Middle East and Africa Cytology Industry Revenue (Million), by Country 2025 & 2033
- Figure 64: Middle East and Africa Cytology Industry Volume (K Unit), by Country 2025 & 2033
- Figure 65: Middle East and Africa Cytology Industry Revenue Share (%), by Country 2025 & 2033
- Figure 66: Middle East and Africa Cytology Industry Volume Share (%), by Country 2025 & 2033
- Figure 67: South America Cytology Industry Revenue (Million), by Type of Examination 2025 & 2033
- Figure 68: South America Cytology Industry Volume (K Unit), by Type of Examination 2025 & 2033
- Figure 69: South America Cytology Industry Revenue Share (%), by Type of Examination 2025 & 2033
- Figure 70: South America Cytology Industry Volume Share (%), by Type of Examination 2025 & 2033
- Figure 71: South America Cytology Industry Revenue (Million), by Test Type 2025 & 2033
- Figure 72: South America Cytology Industry Volume (K Unit), by Test Type 2025 & 2033
- Figure 73: South America Cytology Industry Revenue Share (%), by Test Type 2025 & 2033
- Figure 74: South America Cytology Industry Volume Share (%), by Test Type 2025 & 2033
- Figure 75: South America Cytology Industry Revenue (Million), by End User 2025 & 2033
- Figure 76: South America Cytology Industry Volume (K Unit), by End User 2025 & 2033
- Figure 77: South America Cytology Industry Revenue Share (%), by End User 2025 & 2033
- Figure 78: South America Cytology Industry Volume Share (%), by End User 2025 & 2033
- Figure 79: South America Cytology Industry Revenue (Million), by Country 2025 & 2033
- Figure 80: South America Cytology Industry Volume (K Unit), by Country 2025 & 2033
- Figure 81: South America Cytology Industry Revenue Share (%), by Country 2025 & 2033
- Figure 82: South America Cytology Industry Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Cytology Industry Revenue Million Forecast, by Type of Examination 2020 & 2033
- Table 2: Global Cytology Industry Volume K Unit Forecast, by Type of Examination 2020 & 2033
- Table 3: Global Cytology Industry Revenue Million Forecast, by Test Type 2020 & 2033
- Table 4: Global Cytology Industry Volume K Unit Forecast, by Test Type 2020 & 2033
- Table 5: Global Cytology Industry Revenue Million Forecast, by End User 2020 & 2033
- Table 6: Global Cytology Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 7: Global Cytology Industry Revenue Million Forecast, by Region 2020 & 2033
- Table 8: Global Cytology Industry Volume K Unit Forecast, by Region 2020 & 2033
- Table 9: Global Cytology Industry Revenue Million Forecast, by Type of Examination 2020 & 2033
- Table 10: Global Cytology Industry Volume K Unit Forecast, by Type of Examination 2020 & 2033
- Table 11: Global Cytology Industry Revenue Million Forecast, by Test Type 2020 & 2033
- Table 12: Global Cytology Industry Volume K Unit Forecast, by Test Type 2020 & 2033
- Table 13: Global Cytology Industry Revenue Million Forecast, by End User 2020 & 2033
- Table 14: Global Cytology Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 15: Global Cytology Industry Revenue Million Forecast, by Country 2020 & 2033
- Table 16: Global Cytology Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 17: United States Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 18: United States Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 19: Canada Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 20: Canada Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 21: Mexico Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 22: Mexico Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 23: Global Cytology Industry Revenue Million Forecast, by Type of Examination 2020 & 2033
- Table 24: Global Cytology Industry Volume K Unit Forecast, by Type of Examination 2020 & 2033
- Table 25: Global Cytology Industry Revenue Million Forecast, by Test Type 2020 & 2033
- Table 26: Global Cytology Industry Volume K Unit Forecast, by Test Type 2020 & 2033
- Table 27: Global Cytology Industry Revenue Million Forecast, by End User 2020 & 2033
- Table 28: Global Cytology Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 29: Global Cytology Industry Revenue Million Forecast, by Country 2020 & 2033
- Table 30: Global Cytology Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 31: Germany Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 32: Germany Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 33: United Kingdom Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 34: United Kingdom Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 35: France Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 36: France Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 37: Italy Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 38: Italy Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 39: Spain Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 40: Spain Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 41: Rest of Europe Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 42: Rest of Europe Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 43: Global Cytology Industry Revenue Million Forecast, by Type of Examination 2020 & 2033
- Table 44: Global Cytology Industry Volume K Unit Forecast, by Type of Examination 2020 & 2033
- Table 45: Global Cytology Industry Revenue Million Forecast, by Test Type 2020 & 2033
- Table 46: Global Cytology Industry Volume K Unit Forecast, by Test Type 2020 & 2033
- Table 47: Global Cytology Industry Revenue Million Forecast, by End User 2020 & 2033
- Table 48: Global Cytology Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 49: Global Cytology Industry Revenue Million Forecast, by Country 2020 & 2033
- Table 50: Global Cytology Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 51: China Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 52: China Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 53: Japan Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 54: Japan Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 55: India Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 56: India Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 57: Australia Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 58: Australia Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 59: South korea Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 60: South korea Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 61: Rest of Asia Pacific Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 62: Rest of Asia Pacific Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 63: Global Cytology Industry Revenue Million Forecast, by Type of Examination 2020 & 2033
- Table 64: Global Cytology Industry Volume K Unit Forecast, by Type of Examination 2020 & 2033
- Table 65: Global Cytology Industry Revenue Million Forecast, by Test Type 2020 & 2033
- Table 66: Global Cytology Industry Volume K Unit Forecast, by Test Type 2020 & 2033
- Table 67: Global Cytology Industry Revenue Million Forecast, by End User 2020 & 2033
- Table 68: Global Cytology Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 69: Global Cytology Industry Revenue Million Forecast, by Country 2020 & 2033
- Table 70: Global Cytology Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 71: GCC Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 72: GCC Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 73: South Africa Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 74: South Africa Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 75: Rest of Middle East and Africa Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 76: Rest of Middle East and Africa Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 77: Global Cytology Industry Revenue Million Forecast, by Type of Examination 2020 & 2033
- Table 78: Global Cytology Industry Volume K Unit Forecast, by Type of Examination 2020 & 2033
- Table 79: Global Cytology Industry Revenue Million Forecast, by Test Type 2020 & 2033
- Table 80: Global Cytology Industry Volume K Unit Forecast, by Test Type 2020 & 2033
- Table 81: Global Cytology Industry Revenue Million Forecast, by End User 2020 & 2033
- Table 82: Global Cytology Industry Volume K Unit Forecast, by End User 2020 & 2033
- Table 83: Global Cytology Industry Revenue Million Forecast, by Country 2020 & 2033
- Table 84: Global Cytology Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 85: Brazil Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 86: Brazil Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 87: Argentina Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 88: Argentina Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 89: Rest of South America Cytology Industry Revenue (Million) Forecast, by Application 2020 & 2033
- Table 90: Rest of South America Cytology Industry Volume (K Unit) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Cytology Industry?
The projected CAGR is approximately 13.50%.
2. Which companies are prominent players in the Cytology Industry?
Key companies in the market include Becton Dickinson and Company, Merck KGaA, F Hoffmann-La Roche Ltd, Hologic Inc, Thermo Fisher Scientific Inc, Trivitron Healthcare, Danaher Corporation, Abbott, PerkinElmer Inc, Sysmex Corporation.
3. What are the main segments of the Cytology Industry?
The market segments include Type of Examination, Test Type, End User.
4. Can you provide details about the market size?
The market size is estimated to be USD 20.54 Million as of 2022.
5. What are some drivers contributing to market growth?
Rising Prevalence of Cancer; Increasing Standardization of Pathological Laboratories; Technological Advancements in Diagnostic and Molecular Techniques.
6. What are the notable trends driving market growth?
Cervical Cancer Segment is Expected to Have a Significant Market Share During the Forecast Period.
7. Are there any restraints impacting market growth?
Safety Issues and Diagnostic Accuracy Issues with Histopathological and Cytopathological Tests; Lack of Awareness among the Public for Diagnostic Tests.
8. Can you provide examples of recent developments in the market?
February 2023: BioReference Health LLC announced today it would be one of the first commercial laboratories to offer the CINtec PLUS Cytology test from Roche Diagnostics. CINtec PLUS Cytology is the only FDA-approved dual-stain triage test for patients who have a positive high-risk human papillomavirus (HPV) result.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Cytology Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Cytology Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Cytology Industry?
To stay informed about further developments, trends, and reports in the Cytology Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
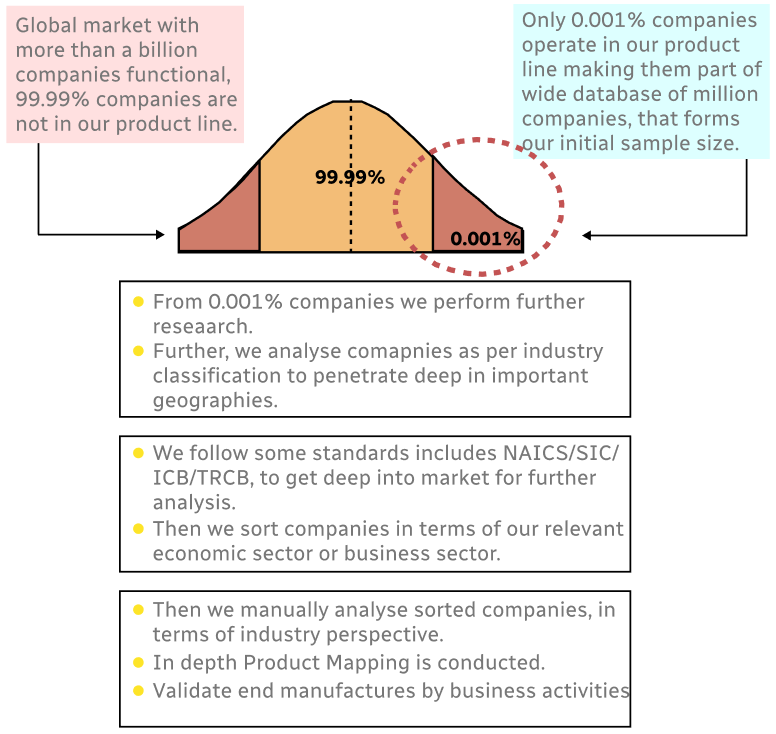
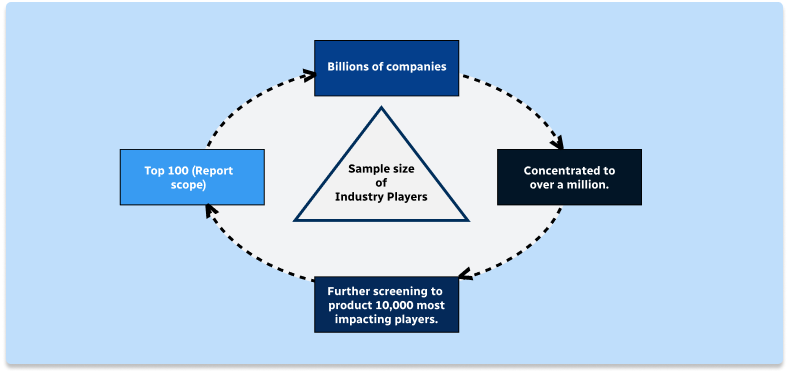
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
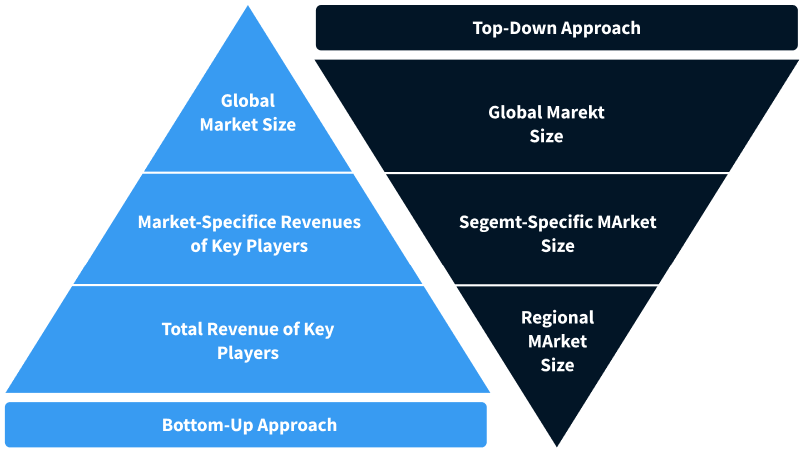
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
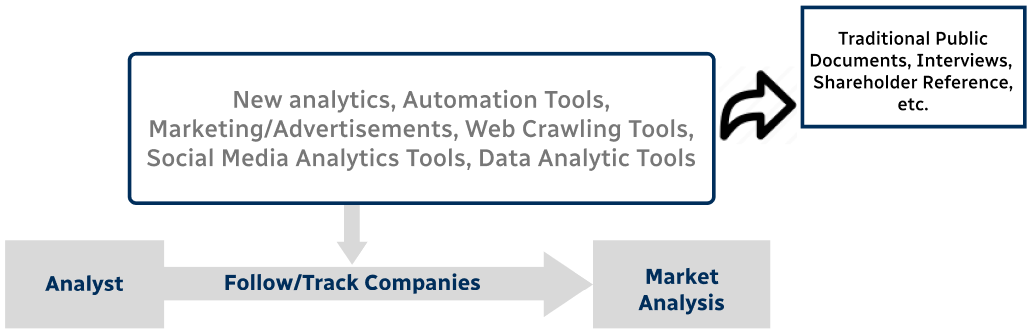
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


