Key Insights
The Italian Minimally Invasive Surgery (MIS) Devices Market is poised for significant expansion, projected to reach $14.76 billion by 2025. This robust growth is underpinned by a Compound Annual Growth Rate (CAGR) of 4.8% during the forecast period of 2025-2033. Several compelling drivers are fueling this upward trajectory. An aging population in Italy, coupled with a rising prevalence of chronic diseases such as cardiovascular and gastrointestinal disorders, is increasing the demand for advanced surgical interventions. Furthermore, a growing preference among both patients and surgeons for less invasive procedures due to their benefits – including reduced recovery times, minimized scarring, and lower complication rates – is a pivotal factor. Technological advancements in MIS devices, encompassing sophisticated handheld instruments, precise guiding devices, advanced electrosurgical tools, and high-definition endoscopic and laparoscopic systems, are continually enhancing surgical outcomes and expanding the scope of MIS applications. The market is characterized by a diverse range of applications, with cardiovascular and gastrointestinal segments demonstrating particularly strong demand, alongside burgeoning interest in gynecological and urological MIS procedures.
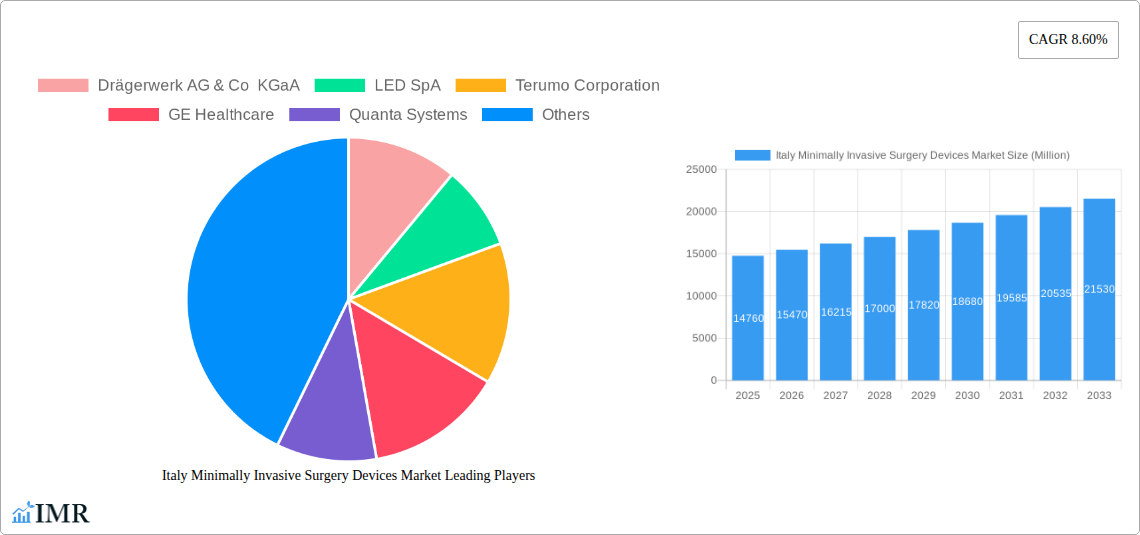
Italy Minimally Invasive Surgery Devices Market Market Size (In Billion)

The Italian MIS Devices Market also benefits from increasing healthcare expenditure and supportive government initiatives aimed at improving healthcare infrastructure and promoting the adoption of advanced medical technologies. Key players such as Medtronic Plc, Olympus Corporation, and Karl Storz GmbH are actively investing in research and development, introducing innovative products, and expanding their distribution networks within Italy. This competitive landscape fosters innovation and drives market penetration across various segments. While the market is experiencing strong growth, potential restraints could include the high initial cost of some advanced MIS equipment and the need for specialized training for surgical teams. However, these challenges are likely to be mitigated by the long-term cost-effectiveness of MIS procedures and ongoing efforts to enhance accessibility. The forecast period is expected to witness sustained innovation, particularly in robotic-assisted MIS and the integration of artificial intelligence for enhanced visualization and precision, further solidifying the market's growth trajectory.
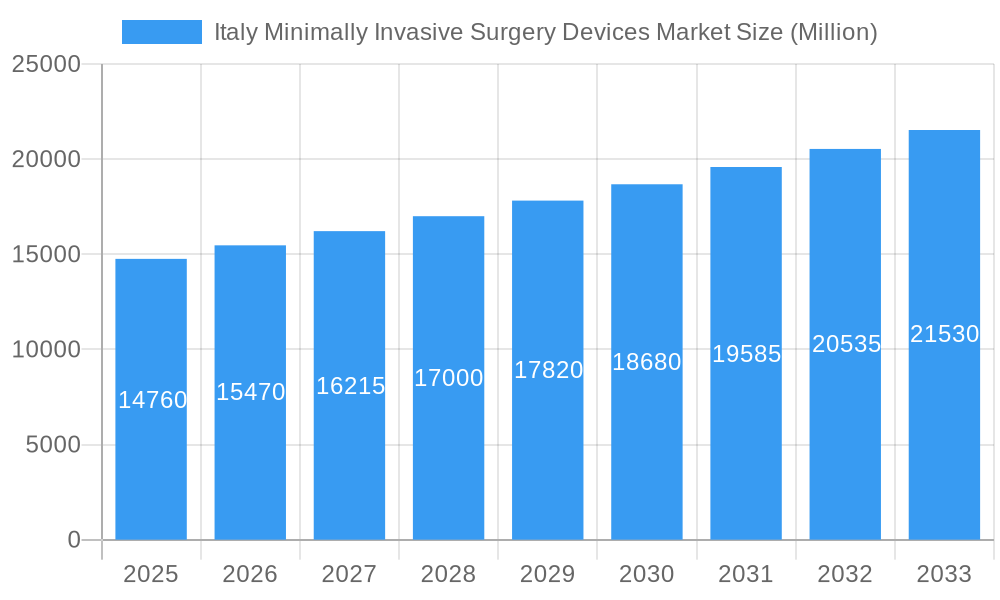
Italy Minimally Invasive Surgery Devices Market Company Market Share

Here's the SEO-optimized report description for the Italy Minimally Invasive Surgery Devices Market, designed for maximum visibility and industry engagement:
Italy Minimally Invasive Surgery Devices Market: Comprehensive Analysis and Forecast (2019–2033)
This in-depth market research report provides a strategic overview of the Italy Minimally Invasive Surgery (MIS) Devices Market, offering critical insights into market dynamics, growth trajectories, and competitive landscapes. Delving into the parent market of global minimally invasive surgery devices and its child market segments, this study leverages data from 2019–2024 (historical) and forecasts for 2025–2033, with 2025 as the base and estimated year. We analyze key product categories, including Handheld Instruments, Guiding Devices, Electrosurgical Devices, Endoscopic Devices, Laparoscopic Devices, Monitoring and Visualization Devices, and Others. Application-wise, the report covers Cardiovascular, Gastrointestinal, Gynecological, Orthopedic, Urological, and Other Applications. For professionals seeking to understand the evolving Italian healthcare technology sector, this report is an indispensable resource.
Italy Minimally Invasive Surgery Devices Market Market Dynamics & Structure
The Italy Minimally Invasive Surgery Devices Market is characterized by a moderate to high concentration, with a few key players holding significant market share. Technological innovation remains a primary driver, fueled by advancements in robotics, artificial intelligence, and miniaturization of surgical instruments. The regulatory framework, overseen by entities like the Italian Ministry of Health and the European Medicines Agency, plays a crucial role in ensuring device safety and efficacy, influencing market entry and product development. Competitive product substitutes, while present, are increasingly being phased out in favor of MIS solutions due to their inherent benefits of reduced patient trauma and faster recovery times. End-user demographics, particularly an aging population and a rising prevalence of chronic diseases requiring surgical intervention, contribute to sustained demand. Mergers and Acquisitions (M&A) trends are observed as companies seek to expand their product portfolios, gain market access, and achieve economies of scale. For instance, strategic partnerships and acquisitions are common, aimed at consolidating market positions. The market's growth is also influenced by the increasing adoption of value-based healthcare models, which prioritize outcomes and cost-effectiveness, favoring minimally invasive approaches.
- Market Concentration: Moderate to High, with prominent global players and specialized local manufacturers.
- Technological Innovation Drivers: Advancements in robotics, AI-assisted surgery, high-definition imaging, and biodegradable materials.
- Regulatory Framework: Stringent compliance with EU MDR (Medical Device Regulation) and national healthcare guidelines.
- Competitive Product Substitutes: Traditional open surgery methods, though their market share is declining.
- End-User Demographics: Growing elderly population, increasing incidence of lifestyle diseases, and demand for reduced hospital stays.
- M&A Trends: Strategic consolidations for portfolio expansion and market penetration.
Italy Minimally Invasive Surgery Devices Market Growth Trends & Insights
The Italy Minimally Invasive Surgery Devices Market is projected for robust growth, with an estimated market size of $XX billion in 2025, projected to reach $YY billion by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of Z.Z% during the forecast period (2025–2033). This expansion is primarily attributed to the increasing adoption rates of minimally invasive techniques across various surgical specialties. Technological disruptions are continuously reshaping the landscape, with the integration of advanced imaging, robotic-assisted surgery, and sophisticated instrumentation enhancing precision and patient outcomes. Consumer behavior shifts are evident, with patients increasingly preferring less invasive procedures due to faster recovery times, reduced pain, and smaller scarring, leading to higher patient demand and physician preference. The growing incidence of chronic diseases, coupled with an aging population, further fuels the demand for surgical interventions where MIS offers significant advantages. The market penetration of MIS devices is steadily increasing as healthcare providers invest in state-of-the-art infrastructure and training to support these advanced surgical modalities. This evolution is supported by continuous research and development efforts from leading manufacturers, focusing on developing more ergonomic, intuitive, and cost-effective MIS solutions. Furthermore, government initiatives aimed at improving healthcare access and quality, alongside reimbursement policies that favor efficient and effective treatments, are acting as significant growth accelerators. The Italian healthcare system's commitment to adopting advanced medical technologies underpins this upward trajectory.
Dominant Regions, Countries, or Segments in Italy Minimally Invasive Surgery Devices Market
Within the Italy Minimally Invasive Surgery Devices Market, the North of Italy region stands out as a dominant force, driven by its superior healthcare infrastructure, higher concentration of specialized medical centers, and a greater propensity for adopting cutting-edge medical technologies. This region boasts a higher per capita expenditure on healthcare and a more developed network of hospitals equipped with advanced surgical suites, making it a prime market for MIS devices. The Cardiovascular application segment is a significant contributor to market growth across Italy, owing to the high prevalence of cardiac diseases and the well-established surgical protocols for minimally invasive cardiac procedures. Furthermore, the Orthopedic and Urological application segments are also experiencing substantial growth, driven by technological advancements in arthroscopy, laparoscopy, and robotic-assisted procedures in these fields. In terms of product segments, Endoscopic Devices and Electrosurgical Devices are leading the market, reflecting their widespread use in various gastrointestinal, gynecological, and urological procedures. The economic policies in the North of Italy, which often incentivize technological adoption and research, coupled with robust infrastructure development, create an environment conducive to market expansion. The presence of leading medical device manufacturers and research institutions within this region further bolsters innovation and market penetration. The high adoption rate of MIS in these specialties, supported by favorable reimbursement policies and growing physician expertise, solidifies the dominance of these segments and the northern region.
- Dominant Region: North of Italy.
- Key Drivers: Advanced healthcare infrastructure, leading medical centers, higher healthcare expenditure, technological adoption.
- Dominant Application Segment: Cardiovascular.
- Key Drivers: High prevalence of cardiac diseases, established MIS cardiac procedures, technological advancements.
- Emerging Application Segments: Orthopedic, Urological.
- Key Drivers: Advancements in arthroscopy, laparoscopy, robotic-assisted surgery.
- Dominant Product Segments: Endoscopic Devices, Electrosurgical Devices.
- Key Drivers: Widespread use in various specialties, continuous product innovation.
Italy Minimally Invasive Surgery Devices Market Product Landscape
The product landscape of the Italy Minimally Invasive Surgery Devices Market is characterized by continuous innovation and a diverse range of offerings. Endoscopic Devices, including advanced visualization systems and robotic endoscopes, are at the forefront, enabling greater precision and tactile feedback during complex procedures. Electrosurgical Devices are evolving with sophisticated monopolar and bipolar technologies, offering enhanced tissue cutting, coagulation, and sealing capabilities with reduced thermal spread. Monitoring and Visualization Devices, such as high-definition monitors, 3D imaging systems, and integrated surgical navigation platforms, are crucial for real-time guidance and improved surgical outcomes. Innovations in Handheld Instruments focus on ergonomic designs, modularity, and enhanced articulation for better maneuverability. The trend towards miniaturization and single-use instruments is also prominent, addressing concerns related to infection control and cost-effectiveness.
Key Drivers, Barriers & Challenges in Italy Minimally Invasive Surgery Devices Market
Key Drivers: The Italy Minimally Invasive Surgery Devices Market is propelled by several factors. Technological advancements in robotics, imaging, and instrumentation continuously enhance surgical capabilities and patient outcomes. An aging population and the increasing prevalence of chronic diseases necessitate more frequent surgical interventions, with MIS offering significant benefits. Growing patient awareness and preference for less invasive procedures, driven by reduced pain and faster recovery, also contribute significantly. Government initiatives and healthcare reforms aimed at improving efficiency and reducing healthcare costs favor the adoption of MIS.
Barriers & Challenges: Despite its growth, the market faces challenges. The high initial cost of advanced MIS equipment, particularly robotic systems, can be a significant barrier for smaller hospitals and clinics. Stringent regulatory approval processes can delay market entry for new devices. A shortage of highly trained surgeons proficient in MIS techniques and the need for extensive training programs also pose challenges. Reimbursement policies, while improving, can still be a hurdle for certain procedures. Furthermore, cybersecurity concerns related to connected surgical devices and data privacy are becoming increasingly important.
Emerging Opportunities in Italy Minimally Invasive Surgery Devices Market
Emerging opportunities in the Italy Minimally Invasive Surgery Devices Market lie in the expansion of robotic-assisted surgery beyond established fields like urology and gynecology into areas such as general surgery and neurosurgery. The development and adoption of AI-powered diagnostic and planning tools that integrate with MIS devices present a significant growth avenue. There is also a growing demand for advanced simulation and training platforms to accelerate surgeon proficiency in MIS. Furthermore, the increasing focus on personalized medicine will drive the need for highly specialized and adaptable MIS instruments tailored to individual patient needs. The development of cost-effective, user-friendly MIS solutions for outpatient settings could also tap into an underserved market.
Growth Accelerators in the Italy Minimally Invasive Surgery Devices Market Industry
Key catalysts driving long-term growth in the Italy Minimally Invasive Surgery Devices Market include continuous technological breakthroughs, such as advancements in miniaturization, haptic feedback, and augmented reality integration in surgical instruments. Strategic partnerships between device manufacturers, healthcare providers, and academic institutions foster innovation and accelerate the translation of research into clinical practice. Market expansion strategies by key players, including the introduction of new product lines and entry into underserved geographical areas within Italy, are crucial. The increasing emphasis on value-based healthcare and the proven cost-effectiveness of MIS in reducing hospital stays and complications further accelerate its adoption and market growth.
Key Players Shaping the Italy Minimally Invasive Surgery Devices Market Market
- Drägerwerk AG & Co KGaA
- LED SpA
- Terumo Corporation
- GE Healthcare
- Quanta Systems
- Karl Storz GmbH
- Fazzini
- Medtronic Plc
- Olympus Corporation
Notable Milestones in Italy Minimally Invasive Surgery Devices Market Sector
- 2020: Increased adoption of remote surgical training and consultation platforms due to the global pandemic, accelerating digital integration in MIS.
- 2021: Launch of next-generation robotic surgical systems with enhanced articulation and AI-driven features, improving precision in complex procedures.
- 2022: Significant investment in the development of single-use MIS instruments to enhance patient safety and reduce infection risks.
- 2023: Expansion of laparoscopic devices into new orthopedic applications, showcasing versatility and growing demand in the segment.
- 2024: Introduction of advanced visualization technologies, including 3D and 4K imaging, further improving surgeon's view and diagnostic capabilities.
In-Depth Italy Minimally Invasive Surgery Devices Market Market Outlook
The future outlook for the Italy Minimally Invasive Surgery Devices Market is exceptionally bright, driven by ongoing technological advancements and a sustained demand for improved patient care. Growth accelerators such as the continuous refinement of robotic-assisted surgery, the integration of artificial intelligence for enhanced surgical planning and execution, and the development of innovative biomaterials for surgical instruments will redefine procedural possibilities. Strategic collaborations between leading medical technology companies and Italian healthcare institutions are poised to foster rapid adoption and clinical validation of novel MIS solutions. The increasing focus on value-based healthcare will further incentivize the shift towards MIS due to its proven ability to deliver superior outcomes while managing costs effectively, thereby unlocking substantial future market potential and presenting lucrative strategic opportunities for stakeholders.
Italy Minimally Invasive Surgery Devices Market Segmentation
-
1. Products
- 1.1. Handheld Instruments
- 1.2. Guiding Devices
- 1.3. Electrosurgical Devices
- 1.4. Endoscopic Devices
- 1.5. Laproscopic Devices
- 1.6. Monitoring and Visualization Devices
- 1.7. Others
-
2. Application
- 2.1. Cardiovascular
- 2.2. Gastrointestinal
- 2.3. Gynecological
- 2.4. Orthopedic
- 2.5. Urological
- 2.6. Other Applications
Italy Minimally Invasive Surgery Devices Market Segmentation By Geography
- 1. Italy
Italy Minimally Invasive Surgery Devices Market Regional Market Share

Geographic Coverage of Italy Minimally Invasive Surgery Devices Market
Italy Minimally Invasive Surgery Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 4.8% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; Rising Number of Surgical Procedures Coupled with Growing Burden of Chronic Disorders; Technological Advancement in Products
- 3.3. Market Restrains
- 3.3.1. ; Shortage of Experienced Professionals
- 3.4. Market Trends
- 3.4.1. Gastrointestinal Segment is Expected to Observe a Significant Growth During the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Italy Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Products
- 5.1.1. Handheld Instruments
- 5.1.2. Guiding Devices
- 5.1.3. Electrosurgical Devices
- 5.1.4. Endoscopic Devices
- 5.1.5. Laproscopic Devices
- 5.1.6. Monitoring and Visualization Devices
- 5.1.7. Others
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Cardiovascular
- 5.2.2. Gastrointestinal
- 5.2.3. Gynecological
- 5.2.4. Orthopedic
- 5.2.5. Urological
- 5.2.6. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Italy
- 5.1. Market Analysis, Insights and Forecast - by Products
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Drägerwerk AG & Co KGaA
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 LED SpA
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Terumo Corporation
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 GE Healthcare
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Quanta Systems
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Karl Storz GmbH
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Fazzini
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Medtronic Plc
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Olympus Corporation
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.1 Drägerwerk AG & Co KGaA
List of Figures
- Figure 1: Italy Minimally Invasive Surgery Devices Market Revenue Breakdown (undefined, %) by Product 2025 & 2033
- Figure 2: Italy Minimally Invasive Surgery Devices Market Share (%) by Company 2025
List of Tables
- Table 1: Italy Minimally Invasive Surgery Devices Market Revenue undefined Forecast, by Products 2020 & 2033
- Table 2: Italy Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Products 2020 & 2033
- Table 3: Italy Minimally Invasive Surgery Devices Market Revenue undefined Forecast, by Application 2020 & 2033
- Table 4: Italy Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Application 2020 & 2033
- Table 5: Italy Minimally Invasive Surgery Devices Market Revenue undefined Forecast, by Region 2020 & 2033
- Table 6: Italy Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Region 2020 & 2033
- Table 7: Italy Minimally Invasive Surgery Devices Market Revenue undefined Forecast, by Products 2020 & 2033
- Table 8: Italy Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Products 2020 & 2033
- Table 9: Italy Minimally Invasive Surgery Devices Market Revenue undefined Forecast, by Application 2020 & 2033
- Table 10: Italy Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Application 2020 & 2033
- Table 11: Italy Minimally Invasive Surgery Devices Market Revenue undefined Forecast, by Country 2020 & 2033
- Table 12: Italy Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Italy Minimally Invasive Surgery Devices Market?
The projected CAGR is approximately 4.8%.
2. Which companies are prominent players in the Italy Minimally Invasive Surgery Devices Market?
Key companies in the market include Drägerwerk AG & Co KGaA, LED SpA, Terumo Corporation, GE Healthcare, Quanta Systems, Karl Storz GmbH, Fazzini, Medtronic Plc, Olympus Corporation.
3. What are the main segments of the Italy Minimally Invasive Surgery Devices Market?
The market segments include Products, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX N/A as of 2022.
5. What are some drivers contributing to market growth?
; Rising Number of Surgical Procedures Coupled with Growing Burden of Chronic Disorders; Technological Advancement in Products.
6. What are the notable trends driving market growth?
Gastrointestinal Segment is Expected to Observe a Significant Growth During the Forecast Period.
7. Are there any restraints impacting market growth?
; Shortage of Experienced Professionals.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in N/A and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Italy Minimally Invasive Surgery Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Italy Minimally Invasive Surgery Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Italy Minimally Invasive Surgery Devices Market?
To stay informed about further developments, trends, and reports in the Italy Minimally Invasive Surgery Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
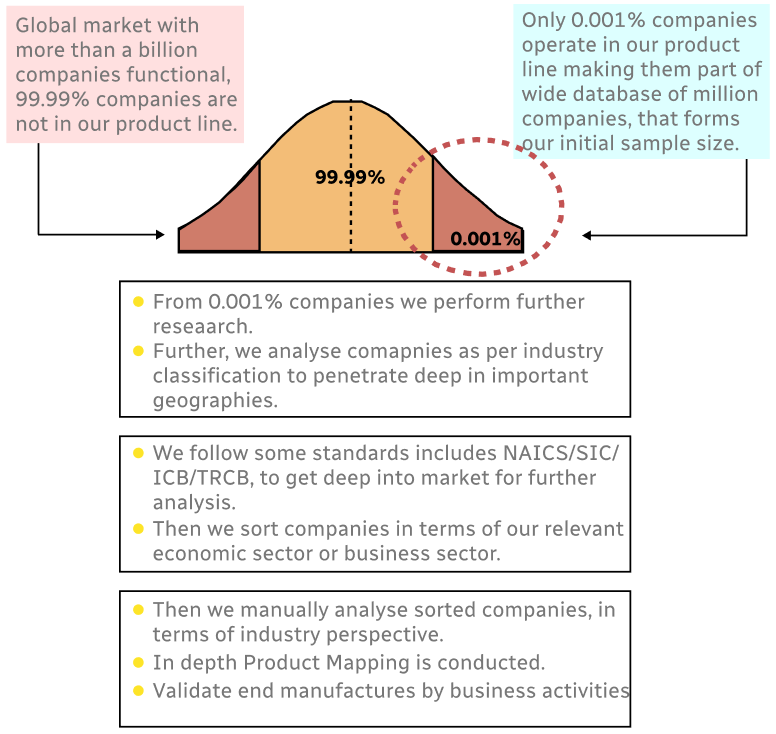
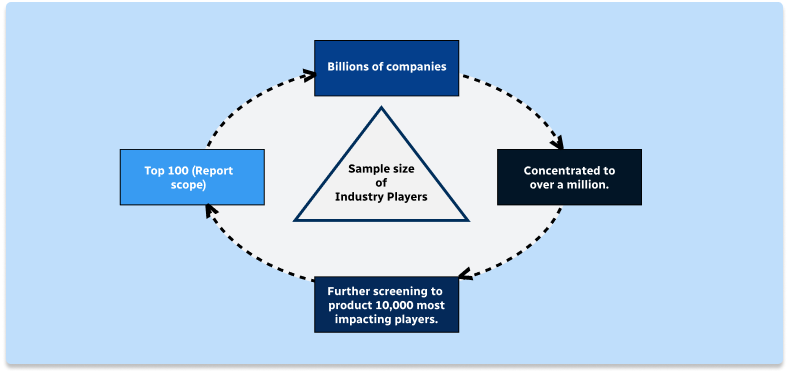
Step 1 - Identification of Relevant Samples Size from Population Database
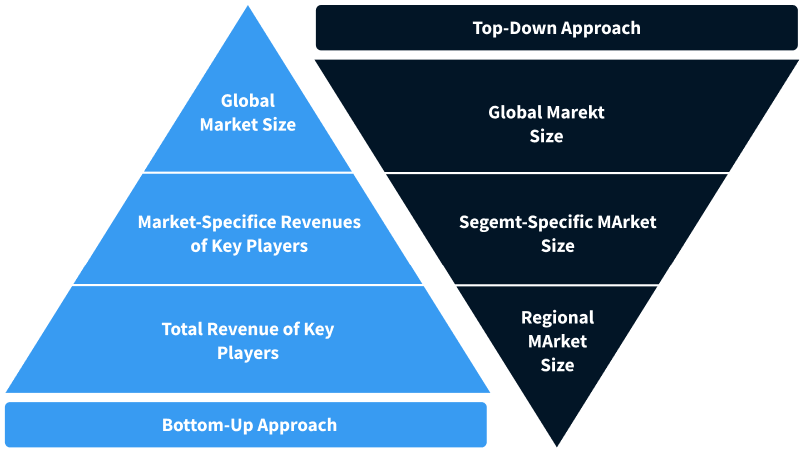
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
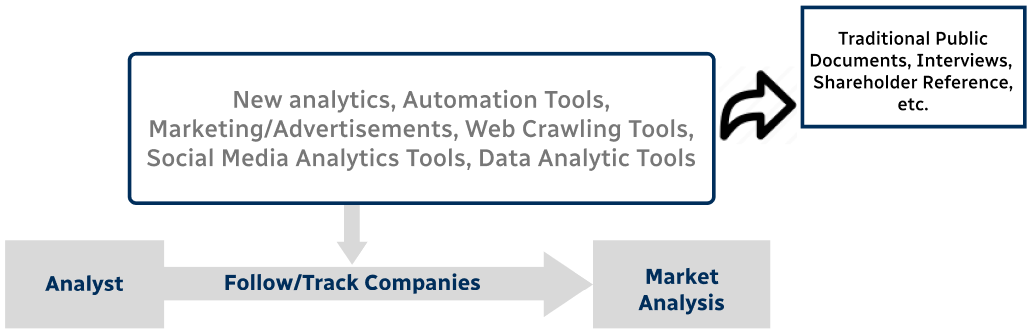
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


