Key Insights
The Middle East & Africa (MEA) In-Vitro Diagnosis (IVD) & Treatment market is poised for significant expansion, projected to reach approximately USD 4.52 million by 2025, with a robust Compound Annual Growth Rate (CAGR) of 4.95% expected to persist through 2033. This growth is propelled by a confluence of factors, including the increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disorders across the region. Enhanced awareness regarding early disease detection and the subsequent demand for accurate diagnostic solutions are acting as major accelerators. Furthermore, advancements in diagnostic technologies, particularly in molecular diagnostics and immunochemistry, are improving diagnostic accuracy and broadening the scope of treatable conditions. The expansion of healthcare infrastructure, coupled with rising disposable incomes in key MEA economies, is also contributing to greater accessibility and adoption of sophisticated IVD solutions.
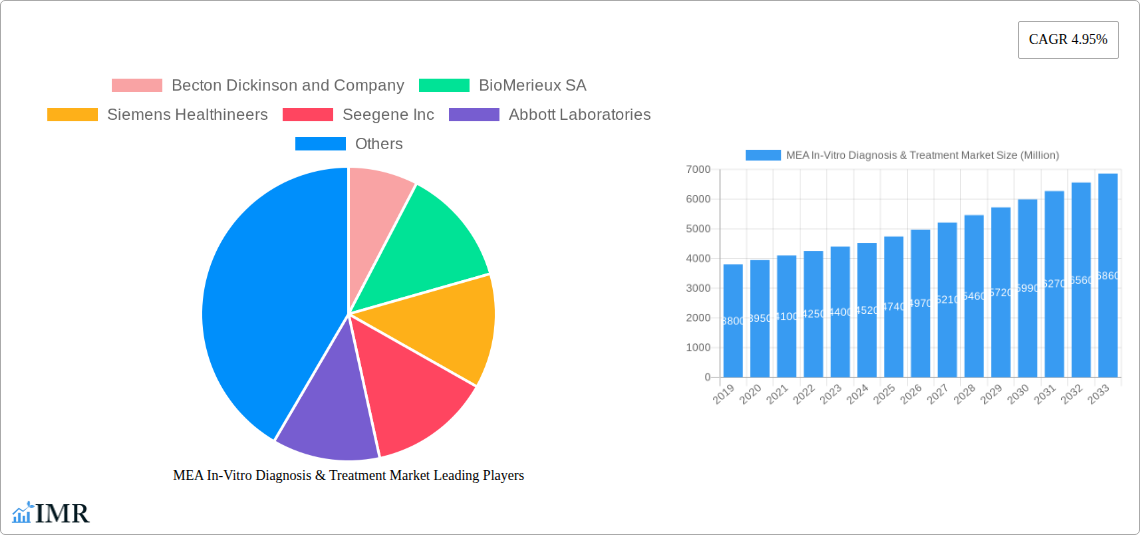
MEA In-Vitro Diagnosis & Treatment Market Market Size (In Billion)

The market segmentation reveals key areas of opportunity and focus. Technologically, Histochemistry and Molecular Diagnostics are anticipated to witness substantial growth, driven by their critical role in personalized medicine and the identification of genetic predispositions. In terms of products, Instruments and Reagents will remain dominant, with increasing emphasis on their integration for streamlined diagnostic workflows. The shift towards Disposable IVD Devices is notable, offering convenience and reducing infection risks, especially in point-of-care settings. Application-wise, Infectious Disease diagnostics, driven by ongoing public health initiatives and pandemic preparedness, along with Diabetes and Cancer/Oncology, will continue to be primary growth drivers. The rising adoption of Point-of-Care Diagnostics, facilitated by mobile health technologies and a focus on decentralized healthcare, presents a transformative trend, enabling faster diagnoses and immediate treatment initiation, particularly beneficial for remote or underserved populations within the MEA region.
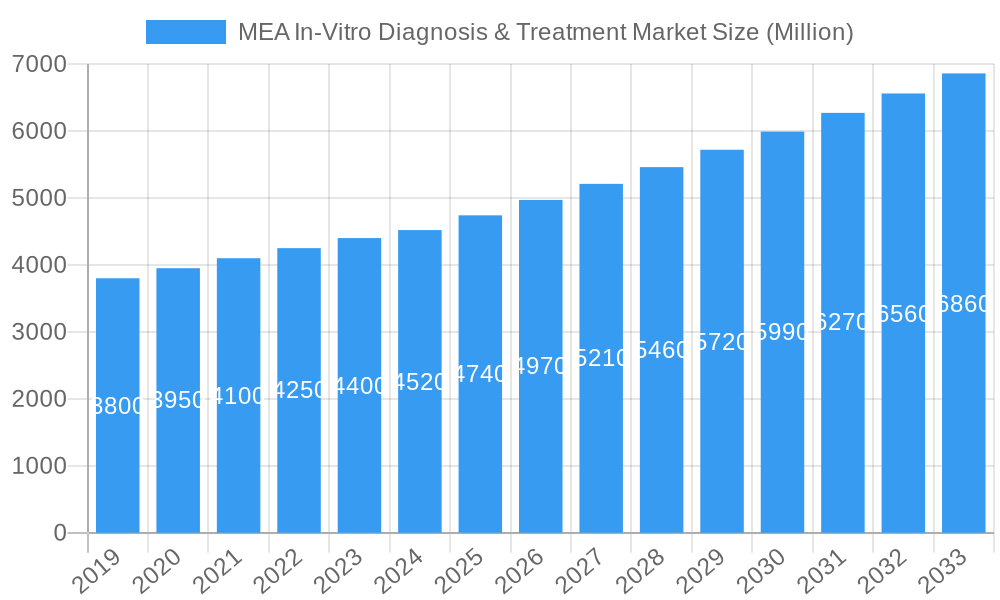
MEA In-Vitro Diagnosis & Treatment Market Company Market Share

Here's a comprehensive, SEO-optimized report description for the MEA In-Vitro Diagnosis & Treatment Market, designed for maximum visibility and engagement.
Report Title: MEA In-Vitro Diagnosis & Treatment Market: Size, Share, Trends, Growth, and Forecast 2025-2033 | Comprehensive Analysis of Key Players, Segments, and Opportunities
Report Description:
Uncover the burgeoning MEA In-Vitro Diagnosis & Treatment Market, a dynamic sector poised for significant expansion driven by increasing healthcare expenditure, rising prevalence of chronic diseases, and rapid technological advancements. This in-depth report offers an exhaustive analysis of the market's current state and future trajectory, providing critical insights for stakeholders seeking to capitalize on emerging opportunities. Explore detailed segmentations including Technique (Molecular Diagnostics, Immunochemistry, Hematology, Histochemistry, Self-blood Glucose Testing), Product (Instruments, Reagents), Usability (Disposable IVD Devices, Reusable IVD Devices), Application (Infectious Diseases, Cancer/Oncology, Diabetes, Cardiology, Autoimmune Diseases), and End-User (Diagnostic Laboratories, Hospitals & Clinics). Gain a strategic advantage by understanding regional dynamics across GCC, South Africa, and the Rest of Middle East & Africa.
This report delves into the intricate Market Dynamics, exploring Technological Innovation Drivers, Regulatory Frameworks, and M&A Trends. Witness the Growth Trends & Insights shaped by evolving Consumer Behavior Shifts and adoption rates. Identify Dominant Regions and Segments fueling market growth, with a granular look at the Product Landscape and Key Drivers, Barriers, and Challenges. Discover Emerging Opportunities and Growth Accelerators, alongside a detailed overview of the Key Players Shaping the Market, including industry giants like F Hoffmann-La Roche AG, Abbott Laboratories, Siemens Healthineers, Thermo Fischer Scientific Inc, Becton Dickinson and Company, Danaher Corporation, BioMerieux SA, Qiagen N V, Sysmex Corporation, and Seegene Inc. This report is your definitive guide to navigating the complex and rewarding MEA In-Vitro Diagnosis & Treatment Market.
MEA In-Vitro Diagnosis & Treatment Market Market Dynamics & Structure
The MEA In-Vitro Diagnosis & Treatment Market is characterized by a moderately consolidated structure, with leading players like F Hoffmann-La Roche AG, Abbott Laboratories, and Siemens Healthineers holding significant market share. Technological innovation serves as a primary driver, with advancements in molecular diagnostics and point-of-care testing revolutionizing diagnostic capabilities. Regulatory frameworks are evolving across the region, with a growing emphasis on standardization and quality control, though variations still exist. Competitive product substitutes are emerging, particularly in the realm of rapid diagnostic tests, offering more accessible and faster results. End-user demographics are shifting towards a greater demand for personalized medicine and early disease detection. Mergers and acquisitions (M&A) are a notable trend, as companies seek to expand their product portfolios and geographic reach within the MEA region. Barriers to innovation include the high cost of advanced diagnostic technologies and limited access to skilled personnel in some sub-regions.
- Market Concentration: Moderate, with a few key global players dominating.
- Technological Innovation Drivers: Miniaturization of instruments, development of novel assay technologies, and integration of AI for data analysis.
- Regulatory Frameworks: Increasing harmonization with international standards, but regional disparities persist.
- Competitive Product Substitutes: Rise of rapid antigen tests, point-of-care devices, and liquid biopsy technologies.
- End-User Demographics: Growing demand for home-use testing and remote patient monitoring solutions.
- M&A Trends: Strategic acquisitions to gain market entry and expand product offerings.
- Innovation Barriers: High capital investment, infrastructure limitations, and regulatory complexities.
MEA In-Vitro Diagnosis & Treatment Market Growth Trends & Insights
The MEA In-Vitro Diagnosis & Treatment Market is experiencing robust growth, projected to witness a Compound Annual Growth Rate (CAGR) of approximately 7.5% during the forecast period of 2025-2033. This expansion is primarily fueled by an increasing healthcare burden from non-communicable diseases, including diabetes and cardiovascular diseases, alongside the persistent threat of infectious diseases. The growing disposable income and a heightened awareness of preventive healthcare among the population are driving higher adoption rates for diagnostic testing. Technological disruptions, such as the widespread implementation of automated laboratory systems and the integration of digital health platforms, are enhancing efficiency and accessibility. Consumer behavior is shifting towards a preference for less invasive diagnostic procedures and readily available diagnostic services, contributing to the demand for disposable IVD devices and point-of-care diagnostics. The market penetration of advanced diagnostic solutions, particularly in segments like molecular diagnostics for pathogen identification and cancer/oncology for early detection and personalized treatment, is a key indicator of this positive trend. The base year, 2025, represents a pivotal point from which these trends are expected to accelerate, with the historical period (2019-2024) laying the groundwork for this forecasted expansion. The estimated market size for 2025 is valued at $3,500 million units, with projections indicating significant future growth.
Dominant Regions, Countries, or Segments in MEA In-Vitro Diagnosis & Treatment Market
The GCC (Gulf Cooperation Council) region stands out as the dominant force within the MEA In-Vitro Diagnosis & Treatment Market, driven by substantial government investment in healthcare infrastructure, a high prevalence of lifestyle-related diseases such as diabetes and cardiovascular diseases, and a wealthy population with a growing demand for advanced medical services. Countries like Saudi Arabia and the UAE are leading this charge, boasting state-of-the-art healthcare facilities and a strong focus on preventive medicine. In terms of segments, Molecular Diagnostics is emerging as a key growth driver, propelled by its critical role in infectious disease surveillance, personalized medicine, and oncology. The increasing demand for precise and rapid identification of pathogens, coupled with advancements in genetic testing and cancer diagnostics, positions this technique at the forefront.
- Dominant Region: GCC – characterized by high per capita healthcare spending and government initiatives to enhance diagnostic capabilities.
- Key Countries in GCC: Saudi Arabia, UAE, Qatar – exhibiting significant adoption of advanced IVD technologies.
- Dominant Technique Segment: Molecular Diagnostics – essential for infectious disease outbreak management, companion diagnostics in oncology, and genetic disorder screening.
- Growth Drivers for Molecular Diagnostics: Increasing genomic research, personalized medicine trends, and rapid detection of emerging infectious agents.
- Leading Product Segment: Instruments and Reagents are equally crucial, with a synergistic demand; however, high-value diagnostic instruments often represent a larger initial investment.
- Dominant Application Segment: Infectious Disease and Cancer/Oncology are currently the primary applications, reflecting regional health challenges and advancements in treatment strategies.
- End-User Dominance: Hospitals and Clinics constitute the largest end-user segment, leveraging IVD for patient care and diagnosis.
- Diagnostic Approach: While Centralized Laboratory-based Diagnostics remain significant, Point-of-Care Diagnostics are rapidly gaining traction due to their convenience and speed, especially in remote areas.
MEA In-Vitro Diagnosis & Treatment Market Product Landscape
The MEA In-Vitro Diagnosis & Treatment Market is witnessing a surge in innovative product offerings tailored to address regional healthcare needs. High-throughput instruments capable of processing a large volume of tests efficiently are becoming indispensable in large diagnostic laboratories and hospitals. Concurrently, the development of highly sensitive and specific reagents is crucial for accurate disease detection across various applications, from identifying viral markers to quantifying biomarkers for chronic diseases. Disposable IVD devices, particularly rapid test kits for infectious diseases and blood glucose monitoring, are experiencing strong demand due to their convenience and affordability. The focus is on miniaturized, user-friendly devices that facilitate point-of-care diagnostics, enabling faster turnaround times and improved patient outcomes. Innovations are also centered on enhancing the multiplexing capabilities of assays, allowing for the simultaneous detection of multiple analytes from a single sample.
Key Drivers, Barriers & Challenges in MEA In-Vitro Diagnosis & Treatment Market
Key Drivers:
- Rising Prevalence of Chronic Diseases: Increasing rates of diabetes, cardiovascular diseases, and cancer necessitate advanced diagnostic solutions for early detection and management.
- Growing Healthcare Expenditure: Governments and private entities are investing heavily in healthcare infrastructure and advanced medical technologies.
- Technological Advancements: Innovations in molecular diagnostics, immunochemistry, and point-of-care testing are expanding the scope and accuracy of IVD.
- Increasing Health Awareness: A growing understanding of preventive healthcare and the importance of early diagnosis among the population.
- Government Initiatives: Several MEA countries are focusing on developing robust healthcare systems and promoting medical tourism, which boosts demand for IVD services.
Barriers & Challenges:
- High Cost of Advanced Technologies: The significant investment required for sophisticated IVD equipment and reagents can be a deterrent in some markets.
- Limited Skilled Workforce: A shortage of trained laboratory technicians and specialized medical professionals in certain regions can hinder adoption and effective utilization of IVD.
- Regulatory Variances: Inconsistent regulatory frameworks across different countries within the MEA region can create complexities for market entry and product registration.
- Infrastructure Gaps: Inadequate cold chain logistics and power supply stability in some remote areas can impact the storage and deployment of sensitive diagnostic materials.
- Reimbursement Policies: Inconsistent and sometimes inadequate reimbursement policies for diagnostic tests can affect market accessibility and affordability.
Emerging Opportunities in MEA In-Vitro Diagnosis & Treatment Market
Emerging opportunities in the MEA In-Vitro Diagnosis & Treatment Market are significantly influenced by the burgeoning demand for personalized medicine and companion diagnostics, particularly within the cancer/oncology segment. The push towards integrated healthcare systems presents a fertile ground for the adoption of point-of-care diagnostics, enabling faster decision-making at the patient's bedside. Furthermore, the increasing focus on infectious disease surveillance and rapid response mechanisms opens avenues for advanced molecular diagnostic platforms. The growing elderly population and the rising prevalence of autoimmune diseases are also creating a demand for specialized IVD assays. The untapped potential in some of the less developed nations within the Rest of Middle East & Africa region offers significant opportunities for market penetration, especially for cost-effective and user-friendly diagnostic solutions.
Growth Accelerators in the MEA In-Vitro Diagnosis & Treatment Market Industry
Long-term growth in the MEA In-Vitro Diagnosis & Treatment Market is being accelerated by significant technological breakthroughs, including advancements in artificial intelligence (AI) for diagnostic data interpretation and the development of novel biosensor technologies. Strategic partnerships between global IVD manufacturers and local distributors are crucial for expanding market reach and ensuring effective product support. Market expansion strategies are also being driven by government incentives for local manufacturing and R&D, fostering innovation within the region. The increasing integration of digital health platforms and telemedicine is further accelerating the adoption of remote diagnostics and patient monitoring solutions, thereby expanding the overall market.
Key Players Shaping the MEA In-Vitro Diagnosis & Treatment Market Market
- F Hoffmann-La Roche AG
- Abbott Laboratories
- Siemens Healthineers
- Thermo Fischer Scientific Inc
- Becton Dickinson and Company
- Danaher Corporation
- BioMerieux SA
- Qiagen N V
- Sysmex Corporation
- Seegene Inc
Notable Milestones in MEA In-Vitro Diagnosis & Treatment Market Sector
- March 2022: Audere partnered with Medical Diagnostech (South Africa) to integrate their MD SARS-CoV-2 antigen device with Audere's HealthPulse digital companion app, enhancing digital connectivity in diagnostics.
- February 2022: Trinity Biotech plc received WHO approval for its new HIV screening product, TrinScreen HIV, a rapid fingerstick blood test delivering results in under 12 minutes, demonstrating strong performance in African clinical trials.
In-Depth MEA In-Vitro Diagnosis & Treatment Market Market Outlook
The MEA In-Vitro Diagnosis & Treatment Market is set for sustained and significant growth, propelled by an accelerating adoption of advanced diagnostic technologies and an increasing focus on preventive healthcare. The strategic integration of AI and digital health solutions will further enhance market efficiency and accessibility. Opportunities abound for companies that can offer cost-effective, user-friendly, and region-specific IVD solutions, particularly in infectious disease diagnostics and oncology. The ongoing investments in healthcare infrastructure across the GCC and the increasing demand for advanced medical services in other parts of the region paint a promising picture for future market expansion and the development of innovative healthcare solutions.
MEA In-Vitro Diagnosis & Treatment Market Segmentation
-
1. Technique
- 1.1. Histochemistry
- 1.2. Molecular Diagnostics
- 1.3. Hematology
- 1.4. Self-blood Glucose Testing
- 1.5. Immunochemistry
- 1.6. Other Techniques
-
2. Product
- 2.1. Instrument
- 2.2. Reagent
- 2.3. Other Products
-
3. Usability
- 3.1. Disposable IVD Device
- 3.2. Reusable IVD Device
-
4. Application
- 4.1. Infectious Disease
- 4.2. Diabetes
- 4.3. Cancer/Oncology
- 4.4. Cardiology
- 4.5. Autoimmune Disease
- 4.6. Other Applications
-
5. End User
- 5.1. Diagnostic Laboratories
- 5.2. Hospitals and Clinics
- 5.3. Other End Users (Academic and Medical School)
-
6. Diagnostic Approach
- 6.1. Point-of-Care Diagnostics
- 6.2. Centralized Laboratory-based Diagnostics
-
7. Geography
- 7.1. GCC
- 7.2. South Africa
- 7.3. Rest of Middle East & Africa
MEA In-Vitro Diagnosis & Treatment Market Segmentation By Geography
- 1. GCC
- 2. South Africa
- 3. Rest of Middle East
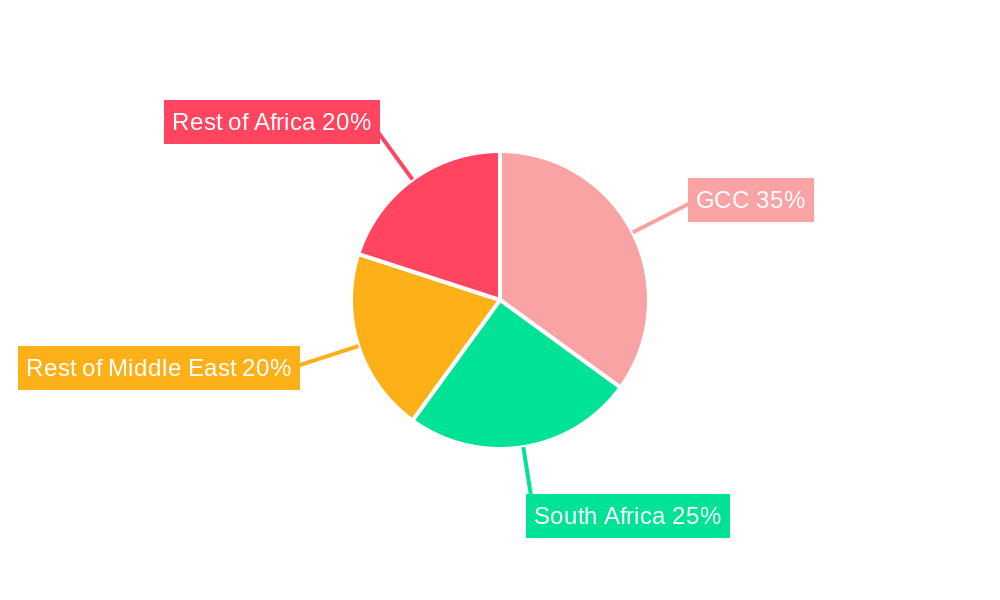
MEA In-Vitro Diagnosis & Treatment Market Regional Market Share

Geographic Coverage of MEA In-Vitro Diagnosis & Treatment Market
MEA In-Vitro Diagnosis & Treatment Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 4.95% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Growing Burden of Chronic Diseases; Increasing Use of Point-of-Care (PoC) Diagnostics; Increasing Use of Advanced Technologies
- 3.3. Market Restrains
- 3.3.1. Stringent Regulations
- 3.4. Market Trends
- 3.4.1. Diabetes Segment is Expected to Register High CAGR in the Application Segment
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global MEA In-Vitro Diagnosis & Treatment Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Technique
- 5.1.1. Histochemistry
- 5.1.2. Molecular Diagnostics
- 5.1.3. Hematology
- 5.1.4. Self-blood Glucose Testing
- 5.1.5. Immunochemistry
- 5.1.6. Other Techniques
- 5.2. Market Analysis, Insights and Forecast - by Product
- 5.2.1. Instrument
- 5.2.2. Reagent
- 5.2.3. Other Products
- 5.3. Market Analysis, Insights and Forecast - by Usability
- 5.3.1. Disposable IVD Device
- 5.3.2. Reusable IVD Device
- 5.4. Market Analysis, Insights and Forecast - by Application
- 5.4.1. Infectious Disease
- 5.4.2. Diabetes
- 5.4.3. Cancer/Oncology
- 5.4.4. Cardiology
- 5.4.5. Autoimmune Disease
- 5.4.6. Other Applications
- 5.5. Market Analysis, Insights and Forecast - by End User
- 5.5.1. Diagnostic Laboratories
- 5.5.2. Hospitals and Clinics
- 5.5.3. Other End Users (Academic and Medical School)
- 5.6. Market Analysis, Insights and Forecast - by Diagnostic Approach
- 5.6.1. Point-of-Care Diagnostics
- 5.6.2. Centralized Laboratory-based Diagnostics
- 5.7. Market Analysis, Insights and Forecast - by Geography
- 5.7.1. GCC
- 5.7.2. South Africa
- 5.7.3. Rest of Middle East & Africa
- 5.8. Market Analysis, Insights and Forecast - by Region
- 5.8.1. GCC
- 5.8.2. South Africa
- 5.8.3. Rest of Middle East
- 5.1. Market Analysis, Insights and Forecast - by Technique
- 6. GCC MEA In-Vitro Diagnosis & Treatment Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Technique
- 6.1.1. Histochemistry
- 6.1.2. Molecular Diagnostics
- 6.1.3. Hematology
- 6.1.4. Self-blood Glucose Testing
- 6.1.5. Immunochemistry
- 6.1.6. Other Techniques
- 6.2. Market Analysis, Insights and Forecast - by Product
- 6.2.1. Instrument
- 6.2.2. Reagent
- 6.2.3. Other Products
- 6.3. Market Analysis, Insights and Forecast - by Usability
- 6.3.1. Disposable IVD Device
- 6.3.2. Reusable IVD Device
- 6.4. Market Analysis, Insights and Forecast - by Application
- 6.4.1. Infectious Disease
- 6.4.2. Diabetes
- 6.4.3. Cancer/Oncology
- 6.4.4. Cardiology
- 6.4.5. Autoimmune Disease
- 6.4.6. Other Applications
- 6.5. Market Analysis, Insights and Forecast - by End User
- 6.5.1. Diagnostic Laboratories
- 6.5.2. Hospitals and Clinics
- 6.5.3. Other End Users (Academic and Medical School)
- 6.6. Market Analysis, Insights and Forecast - by Diagnostic Approach
- 6.6.1. Point-of-Care Diagnostics
- 6.6.2. Centralized Laboratory-based Diagnostics
- 6.7. Market Analysis, Insights and Forecast - by Geography
- 6.7.1. GCC
- 6.7.2. South Africa
- 6.7.3. Rest of Middle East & Africa
- 6.1. Market Analysis, Insights and Forecast - by Technique
- 7. South Africa MEA In-Vitro Diagnosis & Treatment Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Technique
- 7.1.1. Histochemistry
- 7.1.2. Molecular Diagnostics
- 7.1.3. Hematology
- 7.1.4. Self-blood Glucose Testing
- 7.1.5. Immunochemistry
- 7.1.6. Other Techniques
- 7.2. Market Analysis, Insights and Forecast - by Product
- 7.2.1. Instrument
- 7.2.2. Reagent
- 7.2.3. Other Products
- 7.3. Market Analysis, Insights and Forecast - by Usability
- 7.3.1. Disposable IVD Device
- 7.3.2. Reusable IVD Device
- 7.4. Market Analysis, Insights and Forecast - by Application
- 7.4.1. Infectious Disease
- 7.4.2. Diabetes
- 7.4.3. Cancer/Oncology
- 7.4.4. Cardiology
- 7.4.5. Autoimmune Disease
- 7.4.6. Other Applications
- 7.5. Market Analysis, Insights and Forecast - by End User
- 7.5.1. Diagnostic Laboratories
- 7.5.2. Hospitals and Clinics
- 7.5.3. Other End Users (Academic and Medical School)
- 7.6. Market Analysis, Insights and Forecast - by Diagnostic Approach
- 7.6.1. Point-of-Care Diagnostics
- 7.6.2. Centralized Laboratory-based Diagnostics
- 7.7. Market Analysis, Insights and Forecast - by Geography
- 7.7.1. GCC
- 7.7.2. South Africa
- 7.7.3. Rest of Middle East & Africa
- 7.1. Market Analysis, Insights and Forecast - by Technique
- 8. Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Technique
- 8.1.1. Histochemistry
- 8.1.2. Molecular Diagnostics
- 8.1.3. Hematology
- 8.1.4. Self-blood Glucose Testing
- 8.1.5. Immunochemistry
- 8.1.6. Other Techniques
- 8.2. Market Analysis, Insights and Forecast - by Product
- 8.2.1. Instrument
- 8.2.2. Reagent
- 8.2.3. Other Products
- 8.3. Market Analysis, Insights and Forecast - by Usability
- 8.3.1. Disposable IVD Device
- 8.3.2. Reusable IVD Device
- 8.4. Market Analysis, Insights and Forecast - by Application
- 8.4.1. Infectious Disease
- 8.4.2. Diabetes
- 8.4.3. Cancer/Oncology
- 8.4.4. Cardiology
- 8.4.5. Autoimmune Disease
- 8.4.6. Other Applications
- 8.5. Market Analysis, Insights and Forecast - by End User
- 8.5.1. Diagnostic Laboratories
- 8.5.2. Hospitals and Clinics
- 8.5.3. Other End Users (Academic and Medical School)
- 8.6. Market Analysis, Insights and Forecast - by Diagnostic Approach
- 8.6.1. Point-of-Care Diagnostics
- 8.6.2. Centralized Laboratory-based Diagnostics
- 8.7. Market Analysis, Insights and Forecast - by Geography
- 8.7.1. GCC
- 8.7.2. South Africa
- 8.7.3. Rest of Middle East & Africa
- 8.1. Market Analysis, Insights and Forecast - by Technique
- 9. Competitive Analysis
- 9.1. Global Market Share Analysis 2025
- 9.2. Company Profiles
- 9.2.1 Becton Dickinson and Company
- 9.2.1.1. Overview
- 9.2.1.2. Products
- 9.2.1.3. SWOT Analysis
- 9.2.1.4. Recent Developments
- 9.2.1.5. Financials (Based on Availability)
- 9.2.2 BioMerieux SA
- 9.2.2.1. Overview
- 9.2.2.2. Products
- 9.2.2.3. SWOT Analysis
- 9.2.2.4. Recent Developments
- 9.2.2.5. Financials (Based on Availability)
- 9.2.3 Siemens Healthineers
- 9.2.3.1. Overview
- 9.2.3.2. Products
- 9.2.3.3. SWOT Analysis
- 9.2.3.4. Recent Developments
- 9.2.3.5. Financials (Based on Availability)
- 9.2.4 Seegene Inc
- 9.2.4.1. Overview
- 9.2.4.2. Products
- 9.2.4.3. SWOT Analysis
- 9.2.4.4. Recent Developments
- 9.2.4.5. Financials (Based on Availability)
- 9.2.5 Abbott Laboratories
- 9.2.5.1. Overview
- 9.2.5.2. Products
- 9.2.5.3. SWOT Analysis
- 9.2.5.4. Recent Developments
- 9.2.5.5. Financials (Based on Availability)
- 9.2.6 Qiagen N V
- 9.2.6.1. Overview
- 9.2.6.2. Products
- 9.2.6.3. SWOT Analysis
- 9.2.6.4. Recent Developments
- 9.2.6.5. Financials (Based on Availability)
- 9.2.7 Danaher Corporation
- 9.2.7.1. Overview
- 9.2.7.2. Products
- 9.2.7.3. SWOT Analysis
- 9.2.7.4. Recent Developments
- 9.2.7.5. Financials (Based on Availability)
- 9.2.8 F Hoffmann-La Roche AG
- 9.2.8.1. Overview
- 9.2.8.2. Products
- 9.2.8.3. SWOT Analysis
- 9.2.8.4. Recent Developments
- 9.2.8.5. Financials (Based on Availability)
- 9.2.9 Sysmex Corporation
- 9.2.9.1. Overview
- 9.2.9.2. Products
- 9.2.9.3. SWOT Analysis
- 9.2.9.4. Recent Developments
- 9.2.9.5. Financials (Based on Availability)
- 9.2.10 Thermo Fischer Scientific Inc
- 9.2.10.1. Overview
- 9.2.10.2. Products
- 9.2.10.3. SWOT Analysis
- 9.2.10.4. Recent Developments
- 9.2.10.5. Financials (Based on Availability)
- 9.2.1 Becton Dickinson and Company
List of Figures
- Figure 1: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: Global MEA In-Vitro Diagnosis & Treatment Market Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Technique 2025 & 2033
- Figure 4: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Technique 2025 & 2033
- Figure 5: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Technique 2025 & 2033
- Figure 6: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Technique 2025 & 2033
- Figure 7: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Product 2025 & 2033
- Figure 8: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Product 2025 & 2033
- Figure 9: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Product 2025 & 2033
- Figure 10: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Product 2025 & 2033
- Figure 11: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Usability 2025 & 2033
- Figure 12: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Usability 2025 & 2033
- Figure 13: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Usability 2025 & 2033
- Figure 14: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Usability 2025 & 2033
- Figure 15: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Application 2025 & 2033
- Figure 16: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Application 2025 & 2033
- Figure 17: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Application 2025 & 2033
- Figure 18: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Application 2025 & 2033
- Figure 19: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by End User 2025 & 2033
- Figure 20: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by End User 2025 & 2033
- Figure 21: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by End User 2025 & 2033
- Figure 22: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by End User 2025 & 2033
- Figure 23: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Diagnostic Approach 2025 & 2033
- Figure 24: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Diagnostic Approach 2025 & 2033
- Figure 25: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Diagnostic Approach 2025 & 2033
- Figure 26: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Diagnostic Approach 2025 & 2033
- Figure 27: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Geography 2025 & 2033
- Figure 28: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Geography 2025 & 2033
- Figure 29: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Geography 2025 & 2033
- Figure 30: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Geography 2025 & 2033
- Figure 31: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Country 2025 & 2033
- Figure 32: GCC MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Country 2025 & 2033
- Figure 33: GCC MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Country 2025 & 2033
- Figure 34: GCC MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Country 2025 & 2033
- Figure 35: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Technique 2025 & 2033
- Figure 36: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Technique 2025 & 2033
- Figure 37: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Technique 2025 & 2033
- Figure 38: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Technique 2025 & 2033
- Figure 39: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Product 2025 & 2033
- Figure 40: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Product 2025 & 2033
- Figure 41: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Product 2025 & 2033
- Figure 42: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Product 2025 & 2033
- Figure 43: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Usability 2025 & 2033
- Figure 44: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Usability 2025 & 2033
- Figure 45: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Usability 2025 & 2033
- Figure 46: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Usability 2025 & 2033
- Figure 47: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Application 2025 & 2033
- Figure 48: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Application 2025 & 2033
- Figure 49: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Application 2025 & 2033
- Figure 50: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Application 2025 & 2033
- Figure 51: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by End User 2025 & 2033
- Figure 52: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by End User 2025 & 2033
- Figure 53: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by End User 2025 & 2033
- Figure 54: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by End User 2025 & 2033
- Figure 55: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Diagnostic Approach 2025 & 2033
- Figure 56: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Diagnostic Approach 2025 & 2033
- Figure 57: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Diagnostic Approach 2025 & 2033
- Figure 58: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Diagnostic Approach 2025 & 2033
- Figure 59: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Geography 2025 & 2033
- Figure 60: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Geography 2025 & 2033
- Figure 61: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Geography 2025 & 2033
- Figure 62: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Geography 2025 & 2033
- Figure 63: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Country 2025 & 2033
- Figure 64: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Country 2025 & 2033
- Figure 65: South Africa MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Country 2025 & 2033
- Figure 66: South Africa MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Country 2025 & 2033
- Figure 67: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Technique 2025 & 2033
- Figure 68: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Technique 2025 & 2033
- Figure 69: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Technique 2025 & 2033
- Figure 70: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Technique 2025 & 2033
- Figure 71: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Product 2025 & 2033
- Figure 72: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Product 2025 & 2033
- Figure 73: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Product 2025 & 2033
- Figure 74: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Product 2025 & 2033
- Figure 75: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Usability 2025 & 2033
- Figure 76: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Usability 2025 & 2033
- Figure 77: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Usability 2025 & 2033
- Figure 78: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Usability 2025 & 2033
- Figure 79: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Application 2025 & 2033
- Figure 80: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Application 2025 & 2033
- Figure 81: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Application 2025 & 2033
- Figure 82: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Application 2025 & 2033
- Figure 83: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by End User 2025 & 2033
- Figure 84: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by End User 2025 & 2033
- Figure 85: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by End User 2025 & 2033
- Figure 86: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by End User 2025 & 2033
- Figure 87: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Diagnostic Approach 2025 & 2033
- Figure 88: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Diagnostic Approach 2025 & 2033
- Figure 89: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Diagnostic Approach 2025 & 2033
- Figure 90: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Diagnostic Approach 2025 & 2033
- Figure 91: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Geography 2025 & 2033
- Figure 92: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Geography 2025 & 2033
- Figure 93: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Geography 2025 & 2033
- Figure 94: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Geography 2025 & 2033
- Figure 95: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue (Million), by Country 2025 & 2033
- Figure 96: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume (K Unit), by Country 2025 & 2033
- Figure 97: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Revenue Share (%), by Country 2025 & 2033
- Figure 98: Rest of Middle East MEA In-Vitro Diagnosis & Treatment Market Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Technique 2020 & 2033
- Table 2: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Technique 2020 & 2033
- Table 3: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Product 2020 & 2033
- Table 4: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Product 2020 & 2033
- Table 5: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Usability 2020 & 2033
- Table 6: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Usability 2020 & 2033
- Table 7: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Application 2020 & 2033
- Table 8: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Application 2020 & 2033
- Table 9: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by End User 2020 & 2033
- Table 10: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by End User 2020 & 2033
- Table 11: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Diagnostic Approach 2020 & 2033
- Table 12: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Diagnostic Approach 2020 & 2033
- Table 13: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 14: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 15: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Region 2020 & 2033
- Table 16: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Region 2020 & 2033
- Table 17: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Technique 2020 & 2033
- Table 18: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Technique 2020 & 2033
- Table 19: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Product 2020 & 2033
- Table 20: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Product 2020 & 2033
- Table 21: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Usability 2020 & 2033
- Table 22: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Usability 2020 & 2033
- Table 23: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Application 2020 & 2033
- Table 24: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Application 2020 & 2033
- Table 25: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by End User 2020 & 2033
- Table 26: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by End User 2020 & 2033
- Table 27: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Diagnostic Approach 2020 & 2033
- Table 28: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Diagnostic Approach 2020 & 2033
- Table 29: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 30: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 31: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Country 2020 & 2033
- Table 32: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 33: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Technique 2020 & 2033
- Table 34: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Technique 2020 & 2033
- Table 35: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Product 2020 & 2033
- Table 36: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Product 2020 & 2033
- Table 37: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Usability 2020 & 2033
- Table 38: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Usability 2020 & 2033
- Table 39: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Application 2020 & 2033
- Table 40: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Application 2020 & 2033
- Table 41: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by End User 2020 & 2033
- Table 42: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by End User 2020 & 2033
- Table 43: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Diagnostic Approach 2020 & 2033
- Table 44: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Diagnostic Approach 2020 & 2033
- Table 45: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 46: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 47: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Country 2020 & 2033
- Table 48: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Country 2020 & 2033
- Table 49: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Technique 2020 & 2033
- Table 50: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Technique 2020 & 2033
- Table 51: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Product 2020 & 2033
- Table 52: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Product 2020 & 2033
- Table 53: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Usability 2020 & 2033
- Table 54: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Usability 2020 & 2033
- Table 55: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Application 2020 & 2033
- Table 56: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Application 2020 & 2033
- Table 57: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by End User 2020 & 2033
- Table 58: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by End User 2020 & 2033
- Table 59: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Diagnostic Approach 2020 & 2033
- Table 60: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Diagnostic Approach 2020 & 2033
- Table 61: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Geography 2020 & 2033
- Table 62: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Geography 2020 & 2033
- Table 63: Global MEA In-Vitro Diagnosis & Treatment Market Revenue Million Forecast, by Country 2020 & 2033
- Table 64: Global MEA In-Vitro Diagnosis & Treatment Market Volume K Unit Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the MEA In-Vitro Diagnosis & Treatment Market?
The projected CAGR is approximately 4.95%.
2. Which companies are prominent players in the MEA In-Vitro Diagnosis & Treatment Market?
Key companies in the market include Becton Dickinson and Company, BioMerieux SA, Siemens Healthineers, Seegene Inc, Abbott Laboratories, Qiagen N V, Danaher Corporation, F Hoffmann-La Roche AG, Sysmex Corporation, Thermo Fischer Scientific Inc.
3. What are the main segments of the MEA In-Vitro Diagnosis & Treatment Market?
The market segments include Technique, Product, Usability, Application, End User, Diagnostic Approach, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD 4.52 Million as of 2022.
5. What are some drivers contributing to market growth?
Growing Burden of Chronic Diseases; Increasing Use of Point-of-Care (PoC) Diagnostics; Increasing Use of Advanced Technologies.
6. What are the notable trends driving market growth?
Diabetes Segment is Expected to Register High CAGR in the Application Segment.
7. Are there any restraints impacting market growth?
Stringent Regulations.
8. Can you provide examples of recent developments in the market?
March 2022: Audere entered into a partnership with Medical Diagnostech, headquartered in South Africa, a developer, and manufacturer of lateral flow rapid diagnostic test kits. This partnership will pair Medical Diagnostech's MD SARS-CoV-2 antigen device with Audere's HealthPulse digital companion app.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "MEA In-Vitro Diagnosis & Treatment Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the MEA In-Vitro Diagnosis & Treatment Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the MEA In-Vitro Diagnosis & Treatment Market?
To stay informed about further developments, trends, and reports in the MEA In-Vitro Diagnosis & Treatment Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
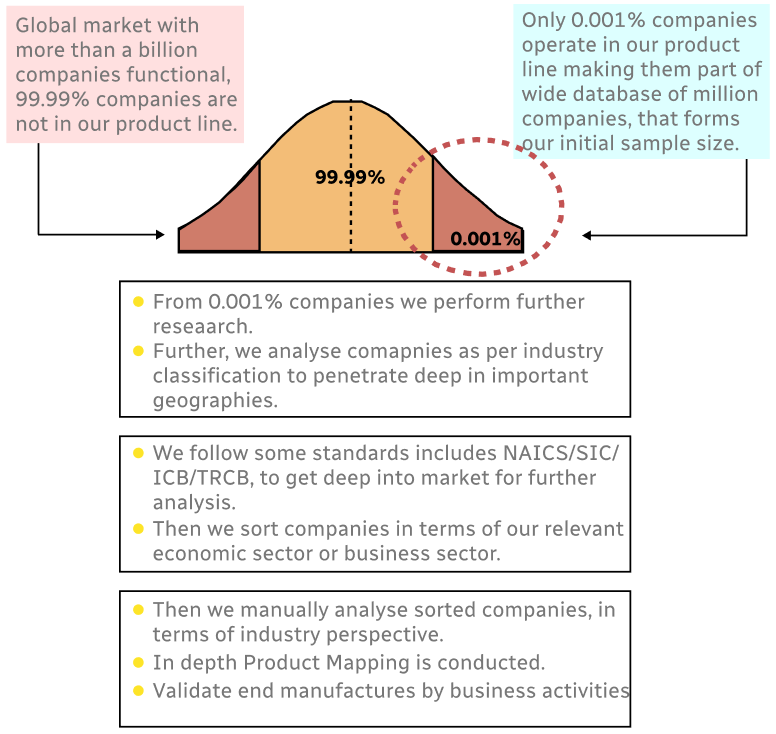
Step 1 - Identification of Relevant Samples Size from Population Database
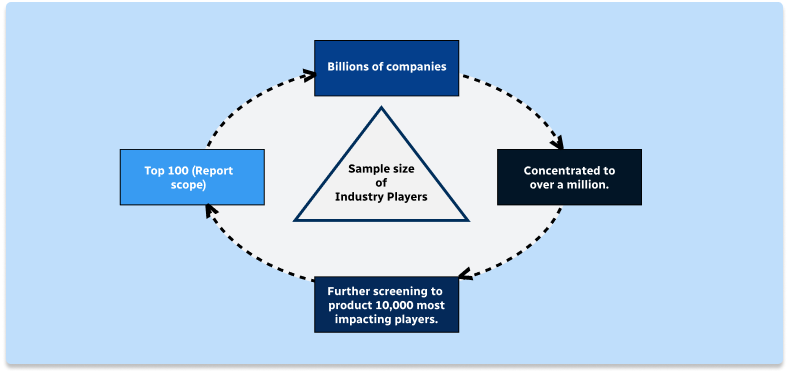
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
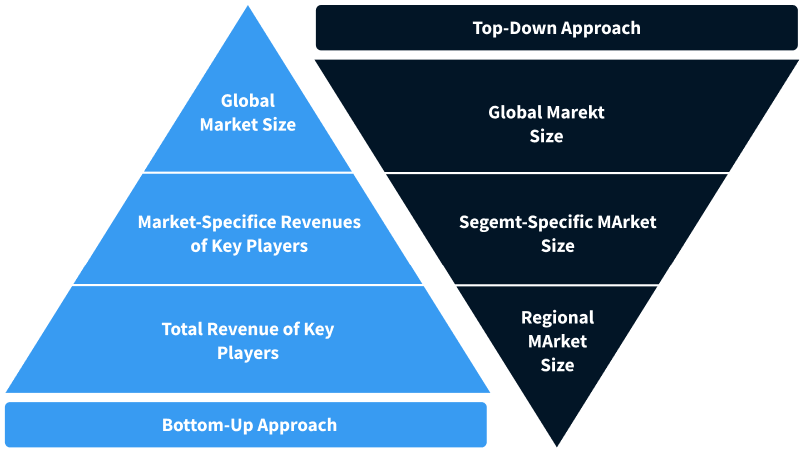
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


