Key Insights
The France spinal surgery devices market is poised for significant expansion, projected to reach $14.7 billion by 2025, driven by an anticipated CAGR of 6% throughout the forecast period. This robust growth is largely propelled by an increasing prevalence of spinal disorders, including degenerative disc disease, scoliosis, and spinal stenosis, which are becoming more common with an aging population and sedentary lifestyles. Advancements in minimally invasive surgical techniques and the development of innovative spinal implants and devices are also key drivers, enabling surgeons to perform complex procedures with greater precision, reduced patient trauma, and faster recovery times. The demand for advanced spinal decompression and fusion devices, in particular, is expected to remain high as healthcare providers and patients alike seek effective solutions for chronic back pain and spinal deformities.
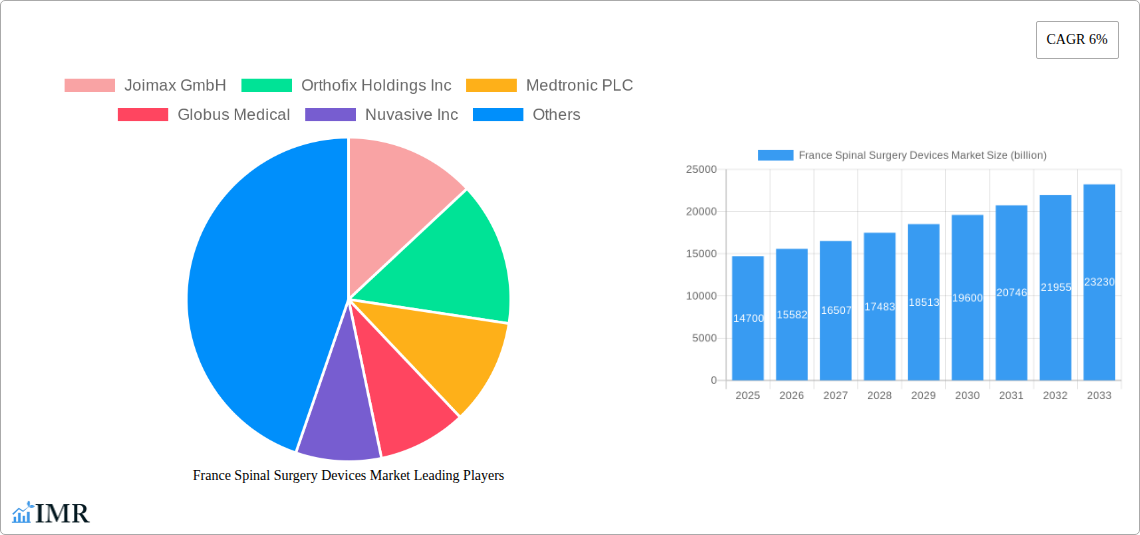
France Spinal Surgery Devices Market Market Size (In Billion)

Further fueling this growth are emerging trends such as the increasing adoption of robotic-assisted spinal surgery, which enhances accuracy and reduces surgeon fatigue, and the development of bio-compatible and absorbable materials for spinal implants, promising improved patient outcomes and reduced complications. The market is segmented into crucial categories including Spinal Decompression (Corpectomy, Discectomy, Facetectomy, Foraminotomy, Laminotomy), Spinal Fusion (Cervical Fusion, Interbody Fusion, ThoracoLumbar Fusion, Other Spinal Fusions), Fracture Repair Devices, Arthroplasty Devices, Non-fusion Devices, and Other Fracture Repair Devices, each contributing to the overall market dynamism. Leading companies such as Medtronic PLC, Johnson & Johnson, Stryker Corporation, Zimmer Biomet, Globus Medical, and NuVasive Inc. are actively investing in research and development to launch next-generation spinal technologies, further stimulating market expansion within France.
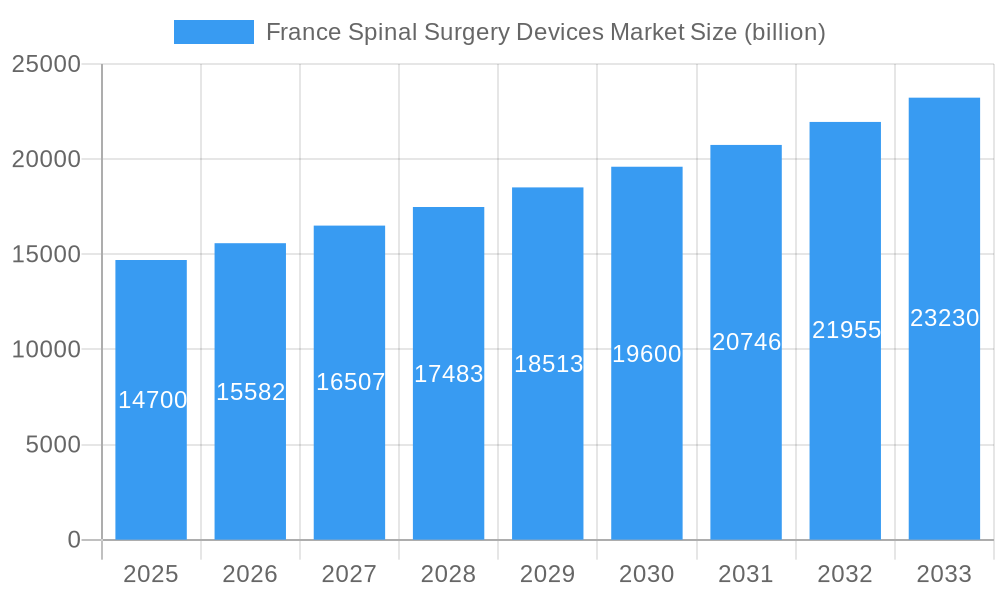
France Spinal Surgery Devices Market Company Market Share

This in-depth report provides a comprehensive analysis of the France Spinal Surgery Devices Market, offering critical insights for industry professionals, investors, and stakeholders. Covering the historical period from 2019 to 2024 and projecting growth through 2033, this study delves into market dynamics, growth trends, product landscape, and key players shaping the future of spinal surgery in France. Our analysis integrates high-traffic SEO keywords to ensure maximum visibility for crucial information on spinal decompression, spinal fusion, fracture repair, arthroplasty, and non-fusion devices.
France Spinal Surgery Devices Market Market Dynamics & Structure
The France Spinal Surgery Devices Market is characterized by a moderate market concentration, with a few key global players holding significant market share. Technological innovation is a primary driver, with advancements in minimally invasive surgical techniques and implantable biomaterials continuously shaping the product landscape. The regulatory framework, governed by agencies like the ANSM (Agence nationale de sécurité du médicament et des produits de santé), plays a crucial role in product approvals and market access, often creating stringent yet necessary barriers to entry. Competitive product substitutes, particularly in less invasive or non-surgical treatment options for spinal conditions, present ongoing challenges. End-user demographics, including an aging population with a rising incidence of degenerative spinal diseases and increasing demand for effective pain management solutions, are fueling market growth. Mergers & Acquisitions (M&A) trends are evident as larger companies seek to expand their portfolios and geographical reach, consolidating market power.
- Market Concentration: Dominated by a mix of large multinational corporations and specialized niche players.
- Technological Innovation: Focus on robotics, AI-assisted surgical planning, advanced materials for implants, and biologics.
- Regulatory Environment: Strict adherence to EU MDR (Medical Device Regulation) and national French regulations for product safety and efficacy.
- Competitive Landscape: Intense competition among implant manufacturers, instrument providers, and companies offering alternative treatment modalities.
- End-User Demographics: Increasing prevalence of spinal deformities, osteoporosis, and age-related spinal conditions driving demand.
- M&A Trends: Strategic acquisitions aimed at acquiring innovative technologies and expanding market presence.
France Spinal Surgery Devices Market Growth Trends & Insights
The France Spinal Surgery Devices Market is poised for substantial growth, driven by a confluence of factors including an increasing prevalence of spinal disorders and advancements in surgical technologies. The market size for spinal surgery devices in France is projected to reach XX billion units by the end of the forecast period. Adoption rates for advanced spinal surgery devices, particularly those enabling minimally invasive procedures, are steadily increasing as surgeons and patients recognize their benefits, such as reduced recovery times and improved outcomes. Technological disruptions, including the integration of robotic-assisted surgery and navigation systems, are transforming surgical precision and patient safety, acting as significant growth accelerators. Consumer behavior shifts are also playing a pivotal role, with a growing demand for personalized treatment plans and less invasive alternatives to traditional open surgeries. The rising incidence of degenerative disc disease, scoliosis, and vertebral fractures among the aging French population further bolsters the market. Furthermore, increased healthcare spending and a robust reimbursement framework for spinal procedures contribute to the overall positive growth trajectory. The CAGR for the France Spinal Surgery Devices Market is estimated at XX% during the forecast period.
Dominant Regions, Countries, or Segments in France Spinal Surgery Devices Market
Within the France Spinal Surgery Devices Market, Spinal Fusion emerges as the dominant segment, consistently driving market growth due to its efficacy in treating a wide range of spinal conditions requiring long-term stability. This includes significant contributions from Interbody Fusion techniques, which are increasingly favored for their ability to restore disc height and decompress nerve roots, leading to enhanced patient outcomes. The sub-segment of Thoracolumbar Fusion is particularly strong, reflecting the high prevalence of degenerative conditions and trauma in this region of the spine.
- Spinal Fusion: This segment is a primary growth engine, encompassing:
- Interbody Fusion: High adoption due to its effectiveness in treating degenerative disc disease and spinal stenosis. Market share estimated at XX%.
- Thoracolumbar Fusion: Driven by a high incidence of injuries and degenerative conditions in the mid and lower back.
- Cervical Fusion: Essential for addressing neck pain, radiculopathy, and instability.
- Spinal Decompression: While a crucial component of many spinal surgeries, its standalone market share is often intertwined with fusion procedures.
- Discectomy: A common procedure for herniated discs, contributing steadily to the market.
- Laminectomy/Facetectomy: Frequently performed to alleviate nerve compression.
- Fracture Repair Devices: Significant demand due to an aging population and increased incidence of osteoporotic fractures. Market share estimated at XX%.
- Arthroplasty Devices: An emerging area with growing adoption for motion-preserving solutions, though still a smaller segment compared to fusion.
- Non-fusion Devices: Representing innovative alternatives to traditional fusion, these devices are gaining traction but are yet to capture substantial market share.
The dominance of the Spinal Fusion segment is further supported by favorable economic policies that encourage advanced surgical interventions, robust healthcare infrastructure facilitating access to these procedures, and ongoing research and development leading to improved fusion techniques and biomaterials. The growth potential within this segment remains exceptionally high, as it addresses chronic pain and mobility issues effectively.
France Spinal Surgery Devices Market Product Landscape
The product landscape for spinal surgery devices in France is characterized by continuous innovation, focusing on enhancing surgical precision, patient safety, and recovery. Key product categories include advanced spinal decompression devices for minimally invasive procedures, innovative spinal fusion systems utilizing novel biomaterials and PEEK implants for better fusion rates, and next-generation fracture repair devices offering superior stability and bone integration. Applications range from treating complex degenerative conditions and deformities to managing acute spinal trauma. Unique selling propositions often lie in the biocompatibility of materials, reduced profile of implants for less tissue disruption, and enhanced radiolucency for improved post-operative imaging. Technological advancements are seen in the integration of 3D printing for custom implants and the development of bioabsorbable materials.
Key Drivers, Barriers & Challenges in France Spinal Surgery Devices Market
Key Drivers:
The France Spinal Surgery Devices Market is propelled by a confluence of factors. Technologically, advancements in minimally invasive spinal surgery (MISS) techniques and the increasing adoption of robotics and navigation systems are paramount. Economically, rising healthcare expenditure and a favorable reimbursement landscape for spinal procedures in France are significant drivers. Policy-driven factors, including government initiatives promoting advanced healthcare solutions and an aging population with a higher prevalence of spinal disorders, further bolster market growth.
Barriers & Challenges:
Despite strong growth potential, the market faces several barriers. High research and development costs associated with innovative spinal technologies can be prohibitive. Stringent regulatory hurdles for new device approvals require extensive clinical trials and data. Reimbursement limitations for certain advanced or novel procedures can hinder widespread adoption. Intense competition among established players and emerging companies, coupled with the availability of cost-effective alternatives, presents a continuous challenge. Supply chain disruptions and the need for specialized surgeon training also contribute to market restraints.
Emerging Opportunities in France Spinal Surgery Devices Market
Emerging opportunities in the France Spinal Surgery Devices Market lie in the increasing demand for motion-preserving technologies such as artificial disc replacements, offering an alternative to fusion. The untapped potential of biologics and regenerative medicine in enhancing spinal fusion success rates and promoting tissue repair presents a significant avenue. Furthermore, the growing adoption of robotic-assisted surgery in less complex spinal procedures and the development of smart implants with integrated sensors for real-time patient monitoring offer exciting prospects for personalized and data-driven spinal care. Expansion into regional French healthcare centers beyond major metropolitan areas also represents an opportunity for market penetration.
Growth Accelerators in the France Spinal Surgery Devices Market Industry
Catalysts driving long-term growth in the France Spinal Surgery Devices Market include significant technological breakthroughs in areas like AI-powered surgical planning and advanced implant materials that improve fusion rates and patient outcomes. Strategic partnerships between device manufacturers and hospitals for research and development, as well as for training programs, accelerate innovation adoption. Furthermore, market expansion strategies focusing on addressing the unmet needs of specific patient populations, such as the elderly with osteoporosis or younger individuals with sports-related spinal injuries, will fuel sustained growth. The increasing global focus on improving quality of life through effective pain management and restored mobility will continue to be a fundamental growth accelerator.
Key Players Shaping the France Spinal Surgery Devices Market Market
- Joimax GmbH
- Orthofix Holdings Inc
- Medtronic PLC
- Globus Medical
- Nuvasive Inc
- Johnson & Johnson
- Stryker Corporation
- Zimmer Biomet
- SeaSpine Holdings Corporation
- RTI Surgical Holdings Inc
Notable Milestones in France Spinal Surgery Devices Market Sector
- November 2022: Implanet signed an exclusive distribution contract for SMTP Technology Co.'s ultrasonic surgical scalpel in France, strengthening its position in the French medical centers specializing in spine surgery.
- December 2022: Spineart and eCential Robotics entered a long-term collaboration agreement to enhance their offerings in the French and Swiss markets, integrating robotics, surgical navigation, and 2D/3D robotic imaging.
In-Depth France Spinal Surgery Devices Market Market Outlook
The future outlook for the France Spinal Surgery Devices Market is exceptionally positive, driven by a robust pipeline of innovative technologies and an expanding patient base. Growth accelerators such as the ongoing advancements in minimally invasive surgical techniques, the increasing integration of robotics and AI in surgical planning and execution, and the development of superior biomaterials for spinal implants will continue to shape the market. Strategic collaborations between technology providers and healthcare institutions, coupled with a proactive approach to addressing the evolving needs of an aging population, will further solidify France's position as a key market for advanced spinal care solutions. The focus on patient-centric treatments and the pursuit of enhanced surgical outcomes will remain paramount, paving the way for sustained market expansion and value creation.
France Spinal Surgery Devices Market Segmentation
-
1. Device Type
-
1.1. Spinal Decompression
- 1.1.1. Corpectomy
- 1.1.2. Discectomy
- 1.1.3. Facetectomy
- 1.1.4. Foraminotomy
- 1.1.5. Laminotomy
-
1.2. Spinal Fusion
- 1.2.1. Cervical Fusion
- 1.2.2. Interbody Fusion
- 1.2.3. ThoracoLumbar Fusion
- 1.2.4. Other Spinal Fusions
- 1.3. Fracture Repair Devices
- 1.4. Arthroplasty Devices
- 1.5. Non-fusion Devices
- 1.6. Other Fracture Repair Devices
-
1.1. Spinal Decompression
France Spinal Surgery Devices Market Segmentation By Geography
- 1. France
France Spinal Surgery Devices Market Regional Market Share

Geographic Coverage of France Spinal Surgery Devices Market
France Spinal Surgery Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1 Rise in Technological Advances in Spinal Surgery and Increasing Demand for Minimally Invasive Surgical Procedures; Increasing Incidence of Obesity
- 3.2.2 Aging Population
- 3.2.3 and Associated Spine Disorders
- 3.3. Market Restrains
- 3.3.1. Expensive Treatment Procedures and Stringent Reimbursement Concerns
- 3.4. Market Trends
- 3.4.1. Arthroplasty Devices Segment is Expected to Hold Significant Share in the French Spinal Surgery Devices Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. France Spinal Surgery Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Spinal Decompression
- 5.1.1.1. Corpectomy
- 5.1.1.2. Discectomy
- 5.1.1.3. Facetectomy
- 5.1.1.4. Foraminotomy
- 5.1.1.5. Laminotomy
- 5.1.2. Spinal Fusion
- 5.1.2.1. Cervical Fusion
- 5.1.2.2. Interbody Fusion
- 5.1.2.3. ThoracoLumbar Fusion
- 5.1.2.4. Other Spinal Fusions
- 5.1.3. Fracture Repair Devices
- 5.1.4. Arthroplasty Devices
- 5.1.5. Non-fusion Devices
- 5.1.6. Other Fracture Repair Devices
- 5.1.1. Spinal Decompression
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. France
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Joimax GmbH
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Orthofix Holdings Inc
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Medtronic PLC
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Globus Medical
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Nuvasive Inc
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Johnson & Johnson
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Stryker Corporation
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Zimmer Biomet
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 SeaSpine Holdings Corporation
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 RTI Surgical Holdings Inc
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.1 Joimax GmbH
List of Figures
- Figure 1: France Spinal Surgery Devices Market Revenue Breakdown (billion, %) by Product 2025 & 2033
- Figure 2: France Spinal Surgery Devices Market Share (%) by Company 2025
List of Tables
- Table 1: France Spinal Surgery Devices Market Revenue billion Forecast, by Device Type 2020 & 2033
- Table 2: France Spinal Surgery Devices Market Volume K Unit Forecast, by Device Type 2020 & 2033
- Table 3: France Spinal Surgery Devices Market Revenue billion Forecast, by Region 2020 & 2033
- Table 4: France Spinal Surgery Devices Market Volume K Unit Forecast, by Region 2020 & 2033
- Table 5: France Spinal Surgery Devices Market Revenue billion Forecast, by Device Type 2020 & 2033
- Table 6: France Spinal Surgery Devices Market Volume K Unit Forecast, by Device Type 2020 & 2033
- Table 7: France Spinal Surgery Devices Market Revenue billion Forecast, by Country 2020 & 2033
- Table 8: France Spinal Surgery Devices Market Volume K Unit Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the France Spinal Surgery Devices Market?
The projected CAGR is approximately 6%.
2. Which companies are prominent players in the France Spinal Surgery Devices Market?
Key companies in the market include Joimax GmbH, Orthofix Holdings Inc, Medtronic PLC, Globus Medical, Nuvasive Inc, Johnson & Johnson, Stryker Corporation, Zimmer Biomet, SeaSpine Holdings Corporation, RTI Surgical Holdings Inc .
3. What are the main segments of the France Spinal Surgery Devices Market?
The market segments include Device Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 14.7 billion as of 2022.
5. What are some drivers contributing to market growth?
Rise in Technological Advances in Spinal Surgery and Increasing Demand for Minimally Invasive Surgical Procedures; Increasing Incidence of Obesity. Aging Population. and Associated Spine Disorders.
6. What are the notable trends driving market growth?
Arthroplasty Devices Segment is Expected to Hold Significant Share in the French Spinal Surgery Devices Market.
7. Are there any restraints impacting market growth?
Expensive Treatment Procedures and Stringent Reimbursement Concerns.
8. Can you provide examples of recent developments in the market?
November 2022: Implanet signed an exclusive distribution contract for SMTP Technology Co.'s ultrasonic surgical scalpel in France. Implanet is a company specializing in spine surgery, and its foothold is in French medical centers.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in billion and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "France Spinal Surgery Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the France Spinal Surgery Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the France Spinal Surgery Devices Market?
To stay informed about further developments, trends, and reports in the France Spinal Surgery Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
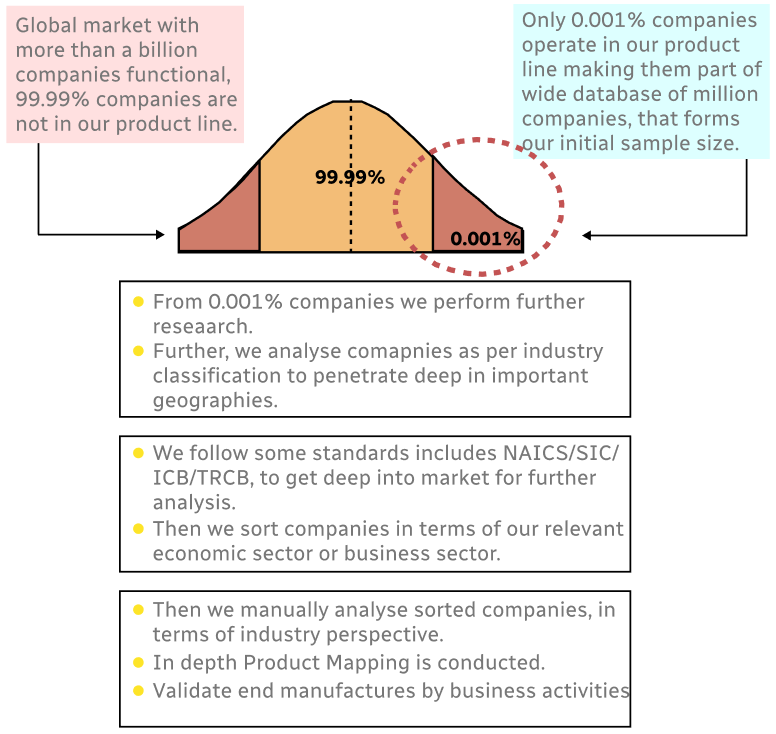
Step 1 - Identification of Relevant Samples Size from Population Database
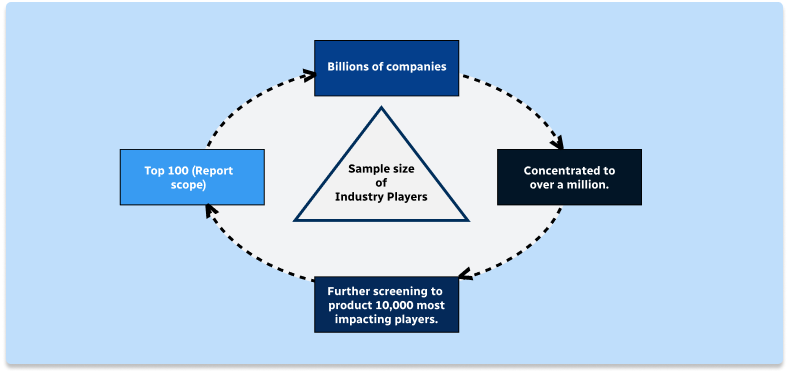
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
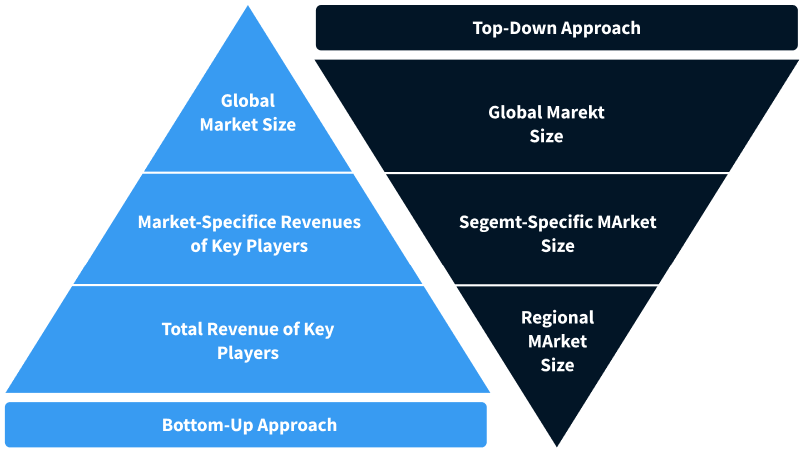
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
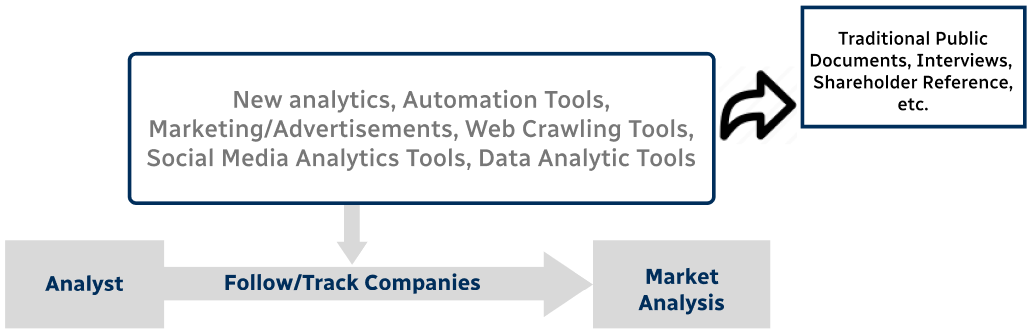
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


