Key Insights
The global Pediatric Vaccines Market is poised for robust expansion, projected to reach a substantial market size of USD 20.29 million and exhibiting a Compound Annual Growth Rate (CAGR) of 6.34% from 2025 to 2033. This significant growth is underpinned by several critical drivers, including increasing parental awareness regarding vaccine-preventable diseases, government initiatives to promote widespread vaccination programs, and advancements in vaccine technologies leading to more effective and safer immunizations. The rising incidence of infectious diseases globally, coupled with a growing emphasis on preventative healthcare strategies for children, further fuels market demand. Key segments driving this expansion include multivalent vaccines, which offer protection against multiple diseases with a single shot, and technologies like live attenuated and inactivated vaccines, which have proven efficacy. The application segment for pneumococcal disease and influenza vaccines, in particular, is expected to witness substantial uptake due to their high prevalence and public health importance. Major market players are actively engaged in research and development, strategic collaborations, and market expansion, contributing to the dynamic landscape of the pediatric vaccine industry.

Pediatric Vaccines Market Market Size (In Million)

While the market enjoys strong growth prospects, certain restraints warrant attention. These include the logistical challenges associated with cold chain management for vaccine distribution, particularly in developing regions, and the persistent issue of vaccine hesitancy fueled by misinformation, which can impact vaccination rates. However, the overarching trend towards strengthening healthcare infrastructure, combined with innovative delivery mechanisms and ongoing public health campaigns, is expected to mitigate these challenges. Geographically, North America and Europe currently lead the market due to well-established healthcare systems and high vaccination coverage. The Asia Pacific region, however, presents a significant growth opportunity, driven by a large pediatric population, increasing disposable incomes, and a growing focus on public health expenditure. Emerging economies within the Middle East and Africa and South America are also anticipated to contribute to market growth as immunization programs expand and access to essential vaccines improves.

Pediatric Vaccines Market Company Market Share

This in-depth report provides a definitive analysis of the global Pediatric Vaccines Market, a critical segment within the broader healthcare industry. Covering the historical period from 2019 to 2024, base and estimated year of 2025, and a comprehensive forecast period extending to 2033, this research offers unparalleled insights into market dynamics, growth trajectories, and competitive landscapes. We delve into the parent market of vaccines and the specialized child vaccine market, providing granular data and expert analysis. Driven by increasing awareness of infectious disease prevention, government immunization programs, and continuous technological advancements in vaccine development, the pediatric vaccines market is poised for significant expansion. The report quantures the market in Million Units, offering precise valuation and volume metrics.
This analysis is essential for pharmaceutical companies, biotechnology firms, public health organizations, investors, and policymakers seeking to understand the evolving demands and opportunities within the children's vaccine market, infant immunization market, and adolescent vaccination market.
Pediatric Vaccines Market Market Dynamics & Structure
The pediatric vaccines market is characterized by a moderately concentrated structure, with a few major global players holding significant market share, alongside emerging companies and regional manufacturers. Technological innovation is a primary driver, with ongoing research into novel vaccine platforms and more effective delivery mechanisms for pediatric populations. Regulatory frameworks, particularly those set by bodies like the FDA and EMA, play a crucial role in dictating product approvals, safety standards, and market access, acting as both an enabler and a potential barrier. Competitive product substitutes are limited due to the highly regulated nature of vaccine development, but alternative disease prevention strategies and evolving vaccination schedules can influence market dynamics. End-user demographics are primarily driven by birth rates, national immunization policies, and public health initiatives aimed at achieving herd immunity. Merger and acquisition (M&A) trends are observed as companies seek to expand their product portfolios, acquire new technologies, or gain a stronger foothold in key geographical markets. For instance, strategic alliances and partnerships are common, aiming to accelerate research and development and broaden market reach. The market's growth is intrinsically linked to the efficacy, safety, and accessibility of vaccines against a wide spectrum of childhood diseases.
- Market Concentration: Dominated by leading pharmaceutical giants, but with increasing opportunities for niche players.
- Technological Innovation Drivers: Development of multi-component vaccines, improved adjuvants, and novel delivery systems.
- Regulatory Frameworks: Stringent approval processes and post-market surveillance by global health authorities.
- Competitive Product Substitutes: Primarily preventive healthcare measures and public health campaigns.
- End-User Demographics: Influenced by global birth rates, childhood disease prevalence, and government vaccination mandates.
- M&A Trends: Focus on portfolio expansion and access to cutting-edge vaccine technologies.
Pediatric Vaccines Market Growth Trends & Insights
The Pediatric Vaccines Market is experiencing robust growth, propelled by an escalating global demand for proactive disease prevention in children. This segment of the broader vaccine market is witnessing a substantial rise in market size, driven by increasing parental awareness regarding the importance of early-life immunization and the efficacy of vaccines in combating serious childhood illnesses. Adoption rates for existing vaccines remain high, bolstered by national immunization programs and vaccination schedules recommended by health organizations worldwide. Technological disruptions are continuously reshaping the landscape, with advancements in vaccine technologies, such as mRNA platforms and innovative adjuvant formulations, promising more effective and safer pediatric vaccines. These innovations are critical for addressing existing unmet needs and developing vaccines against emerging infectious threats. Consumer behavior shifts are evident, with parents increasingly seeking comprehensive vaccination packages and informed healthcare advice. This is further amplified by the accessibility of health information and a growing preference for preventative healthcare measures. The market penetration of critical pediatric vaccines, such as those for pneumococcal disease, influenza, and MMR, is expected to remain high and expand further as access improves in developing regions. The CAGR for the pediatric vaccines market is projected to be robust, reflecting sustained investment in research and development and strong public health support. [Report Placeholder for CAGR Value]% is the projected compound annual growth rate from 2025-2033. The market size in 2025 is estimated at [Report Placeholder for 2025 Market Size in Million Units] Million Units, with a projected growth to [Report Placeholder for Forecasted Market Size in Million Units] Million Units by 2033. Key growth indicators include the declining cost of vaccine production, increasing government funding for immunization campaigns, and the continuous introduction of new vaccines for previously unaddressed pediatric conditions. Furthermore, the expanding global population of children, particularly in emerging economies, provides a substantial and growing customer base for pediatric vaccines. The focus on eradicating vaccine-preventable diseases remains a paramount objective for global health organizations, directly translating into sustained market demand for a wide array of pediatric vaccines.
Dominant Regions, Countries, or Segments in Pediatric Vaccines Market
North America currently stands as a dominant region within the global Pediatric Vaccines Market, driven by robust healthcare infrastructure, high disposable incomes, and strong government initiatives promoting widespread childhood immunization. The United States, in particular, exhibits high adoption rates for a comprehensive range of pediatric vaccines, supported by well-established vaccination schedules and a proactive approach to public health. This dominance is further reinforced by the presence of major pharmaceutical companies headquartered in the region, actively engaged in research, development, and manufacturing of cutting-edge pediatric vaccines.
Within Vaccine Type, Multivalent Vaccines are a significant growth driver. These vaccines offer protection against multiple diseases with a single injection, leading to increased patient compliance and reduced healthcare burdens. The convenience and efficiency of multivalent vaccines make them highly favored in pediatric vaccination programs globally.
In terms of Technology, Conjugate Vaccines are pivotal. These vaccines, particularly effective against bacterial pathogens like Streptococcus pneumoniae, have revolutionized the prevention of serious childhood infections. Their ability to elicit a strong immune response in infants and young children has made them indispensable in pediatric immunization protocols. The development and widespread use of conjugate vaccines have significantly reduced the incidence and mortality associated with pneumococcal diseases in children.
From an Application perspective, Pneumococcal Disease prevention remains a leading segment. The high morbidity and mortality associated with pneumococcal infections in young children have spurred significant investment in pneumococcal conjugate vaccines (PCVs). The continuous refinement of PCVs, including the development of valencies that offer broader protection, contributes to the sustained growth of this segment.
- Dominant Region: North America (USA, Canada)
- Key Drivers: High healthcare expenditure, strong government immunization policies, advanced R&D capabilities, and high public awareness.
- Market Share: Estimated xx% of the global pediatric vaccines market.
- Dominant Vaccine Type: Multivalent Vaccines
- Key Drivers: Convenience, reduced injection frequency, and comprehensive disease protection.
- Growth Potential: Continual development of new combinations addressing a wider spectrum of pathogens.
- Dominant Technology: Conjugate Vaccines
- Key Drivers: High efficacy against bacterial pathogens, strong immune response in infants, and proven track record in disease reduction.
- Market Share: Dominates segments like pneumococcal disease prevention.
- Dominant Application: Pneumococcal Disease
- Key Drivers: High disease burden, widespread vaccination mandates, and ongoing innovation in vaccine formulations (e.g., PCV15, PCV20).
- Market Penetration: High across developed and developing nations.
Pediatric Vaccines Market Product Landscape
The Pediatric Vaccines Market product landscape is characterized by continuous innovation aimed at enhancing efficacy, safety, and patient convenience. Key product developments include the introduction of novel formulations for existing vaccines, as well as the development of vaccines for new infectious agents affecting children. For instance, advancements in Conjugate Vaccine technology have led to the creation of vaccines offering broader serotype coverage for diseases like pneumococcal disease. Furthermore, the exploration of mRNA technology for pediatric applications signals a significant future development. These products are designed to elicit robust immune responses in the delicate physiology of children and infants. Performance metrics are rigorously evaluated through extensive clinical trials, focusing on immunogenicity, safety profiles, and long-term protective effects. Unique selling propositions often revolve around improved protection against a wider range of pathogens, reduced side effects, and simplified administration schedules, such as combination vaccines that protect against multiple diseases in a single shot. The market also sees a growing emphasis on vaccines addressing specific age groups within the pediatric spectrum, from newborns to adolescents.
Key Drivers, Barriers & Challenges in Pediatric Vaccines Market
Key Drivers:
- Government Immunization Programs: Mandates and funding by national health authorities are primary growth accelerators.
- Rising Awareness of Vaccine Preventable Diseases: Increased parental understanding of the critical role of vaccines in child health.
- Technological Advancements: Development of more effective, safer, and broader-spectrum vaccines, including novel platforms.
- Growing Global Child Population: A continuously expanding target demographic for vaccination.
- Eradication Efforts for Infectious Diseases: Global initiatives to eliminate diseases like polio and measles fuel demand.
Barriers & Challenges:
- Vaccine Hesitancy and Misinformation: Concerns, often unfounded, about vaccine safety can impact uptake.
- Stringent Regulatory Approval Processes: Long and costly development cycles for new vaccines.
- Cold Chain Logistics and Infrastructure: Maintaining vaccine efficacy requires a robust and reliable cold chain, particularly in remote or developing regions.
- Pricing Pressures and Affordability: Balancing R&D costs with the need for affordable vaccines, especially for low-income countries.
- Supply Chain Disruptions: Global events can impact the availability of raw materials and the manufacturing capacity for vaccines.
Emerging Opportunities in Pediatric Vaccines Market
Emerging opportunities in the pediatric vaccines market are centered around addressing unmet medical needs and leveraging innovative scientific breakthroughs. The development of vaccines for diseases with significant pediatric impact, such as respiratory syncytial virus (RSV) and new strains of influenza, presents a substantial growth avenue. Furthermore, advancements in personalized medicine and the potential for mRNA technology in pediatric oncology vaccines are areas of significant future exploration. The expansion of vaccination programs into underserved populations and emerging economies, coupled with a growing demand for combination vaccines that simplify immunization schedules, offers considerable market potential. Personalized vaccination strategies based on individual risk factors and genetic predispositions could also emerge as a niche but high-value opportunity.
Growth Accelerators in the Pediatric Vaccines Market Industry
Several catalysts are significantly accelerating the growth of the pediatric vaccines market. Technological breakthroughs in vaccine design, such as the rapid development and deployment of mRNA vaccines, are not only addressing immediate public health needs but also paving the way for future innovations. Strategic partnerships between pharmaceutical giants, academic institutions, and government agencies are crucial for streamlining research, development, and regulatory approval processes. Market expansion strategies, including the penetration of emerging economies and the introduction of vaccines tailored to specific regional disease burdens, are also key growth enablers. Increased global investment in public health infrastructure and a renewed focus on pandemic preparedness are further solidifying the long-term growth trajectory of the pediatric vaccine industry.
Key Players Shaping the Pediatric Vaccines Market Market
- Sanofi SA
- Sinovac Biotech Ltd
- Indian Immunologicals Limited
- Mitsubishi Tanabe Pharma Corporation
- Merck & Co Inc
- Novavax Inc
- Seqirus (CSL Limited)
- AstraZeneca plc
- GlaxoSmithKline PLC
- Pfizer Inc
Notable Milestones in Pediatric Vaccines Market Sector
- June 2022: Pfizer and BioNTech received United States Food and Drug Administration (FDA) emergency use authorization for their COVID-19 vaccine as a three-dose series for children 6 months through 4 years of age. This milestone significantly expanded the pediatric COVID-19 vaccination landscape.
- August 2021: Merck published top-line findings from the pivotal PNEU-PED (V114-029) study. This study evaluated the immunogenicity, safety, and tolerability of VAXNEUVANCE (Pneumococcal 15-valent Conjugate Vaccine) in healthy infants. The trial involved a 4-dose regimen administered at 2, 4, 6, and 12-15 months of age, comparing it against the licensed 13-valent pneumococcal conjugate vaccine (PCV13), highlighting advancements in pneumococcal disease prevention.
In-Depth Pediatric Vaccines Market Market Outlook
The future outlook for the pediatric vaccines market is exceptionally promising, driven by a confluence of factors that promote sustained growth and innovation. The ongoing commitment to eradicating vaccine-preventable diseases globally, coupled with the continuous development of more effective and broadly protective vaccines, ensures a robust demand pipeline. Strategic collaborations and significant investments in research and development are expected to yield new vaccines for emerging infectious threats and currently unaddressed pediatric conditions. Furthermore, the expansion of healthcare access in developing regions and the increasing focus on preventative healthcare measures worldwide will continue to fuel market expansion. The market is poised for significant growth, offering substantial opportunities for stakeholders focused on improving child health through immunization.
Pediatric Vaccines Market Segmentation
-
1. Vaccine Type
- 1.1. Monovalent
- 1.2. Multivalent
-
2. Technology
- 2.1. Live Attenuated
- 2.2. Inactivated
- 2.3. Toxoid
- 2.4. Conjugate
- 2.5. Other Technologies
-
3. Application
- 3.1. Pneumococcal Disease
- 3.2. Influenza
- 3.3. Measles, Mumps, and Rubella (MMR)
- 3.4. Other Applications
Pediatric Vaccines Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America

Pediatric Vaccines Market Regional Market Share

Geographic Coverage of Pediatric Vaccines Market
Pediatric Vaccines Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.34% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Burden of Chronic Diseases with the Importance and Awareness of Immunization; Increase in the Government and Non-government Funding in R&D
- 3.3. Market Restrains
- 3.3.1. Cost of Immunization; Lesser Medical Coverage and Healthcare Services in Low- and Middle-income Countries
- 3.4. Market Trends
- 3.4.1. The Conjugate Vaccine Segment is Expected to Grow at a Significant Rate
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Pediatric Vaccines Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 5.1.1. Monovalent
- 5.1.2. Multivalent
- 5.2. Market Analysis, Insights and Forecast - by Technology
- 5.2.1. Live Attenuated
- 5.2.2. Inactivated
- 5.2.3. Toxoid
- 5.2.4. Conjugate
- 5.2.5. Other Technologies
- 5.3. Market Analysis, Insights and Forecast - by Application
- 5.3.1. Pneumococcal Disease
- 5.3.2. Influenza
- 5.3.3. Measles, Mumps, and Rubella (MMR)
- 5.3.4. Other Applications
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Middle East and Africa
- 5.4.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 6. North America Pediatric Vaccines Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 6.1.1. Monovalent
- 6.1.2. Multivalent
- 6.2. Market Analysis, Insights and Forecast - by Technology
- 6.2.1. Live Attenuated
- 6.2.2. Inactivated
- 6.2.3. Toxoid
- 6.2.4. Conjugate
- 6.2.5. Other Technologies
- 6.3. Market Analysis, Insights and Forecast - by Application
- 6.3.1. Pneumococcal Disease
- 6.3.2. Influenza
- 6.3.3. Measles, Mumps, and Rubella (MMR)
- 6.3.4. Other Applications
- 6.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 7. Europe Pediatric Vaccines Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 7.1.1. Monovalent
- 7.1.2. Multivalent
- 7.2. Market Analysis, Insights and Forecast - by Technology
- 7.2.1. Live Attenuated
- 7.2.2. Inactivated
- 7.2.3. Toxoid
- 7.2.4. Conjugate
- 7.2.5. Other Technologies
- 7.3. Market Analysis, Insights and Forecast - by Application
- 7.3.1. Pneumococcal Disease
- 7.3.2. Influenza
- 7.3.3. Measles, Mumps, and Rubella (MMR)
- 7.3.4. Other Applications
- 7.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 8. Asia Pacific Pediatric Vaccines Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 8.1.1. Monovalent
- 8.1.2. Multivalent
- 8.2. Market Analysis, Insights and Forecast - by Technology
- 8.2.1. Live Attenuated
- 8.2.2. Inactivated
- 8.2.3. Toxoid
- 8.2.4. Conjugate
- 8.2.5. Other Technologies
- 8.3. Market Analysis, Insights and Forecast - by Application
- 8.3.1. Pneumococcal Disease
- 8.3.2. Influenza
- 8.3.3. Measles, Mumps, and Rubella (MMR)
- 8.3.4. Other Applications
- 8.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 9. Middle East and Africa Pediatric Vaccines Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 9.1.1. Monovalent
- 9.1.2. Multivalent
- 9.2. Market Analysis, Insights and Forecast - by Technology
- 9.2.1. Live Attenuated
- 9.2.2. Inactivated
- 9.2.3. Toxoid
- 9.2.4. Conjugate
- 9.2.5. Other Technologies
- 9.3. Market Analysis, Insights and Forecast - by Application
- 9.3.1. Pneumococcal Disease
- 9.3.2. Influenza
- 9.3.3. Measles, Mumps, and Rubella (MMR)
- 9.3.4. Other Applications
- 9.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 10. South America Pediatric Vaccines Market Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 10.1.1. Monovalent
- 10.1.2. Multivalent
- 10.2. Market Analysis, Insights and Forecast - by Technology
- 10.2.1. Live Attenuated
- 10.2.2. Inactivated
- 10.2.3. Toxoid
- 10.2.4. Conjugate
- 10.2.5. Other Technologies
- 10.3. Market Analysis, Insights and Forecast - by Application
- 10.3.1. Pneumococcal Disease
- 10.3.2. Influenza
- 10.3.3. Measles, Mumps, and Rubella (MMR)
- 10.3.4. Other Applications
- 10.1. Market Analysis, Insights and Forecast - by Vaccine Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Sanofi SA
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Sinovac Biotech Ltd
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Indian Immunologicals Limited
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Mitsubishi Tanabe Pharma Corporation
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Merck & Co Inc
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Novavax Inc *List Not Exhaustive
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Seqirus (CSL Limited)
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 AstraZeneca plc
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 GlaxoSmithKline PLC
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Pfizer Inc
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.1 Sanofi SA
List of Figures
- Figure 1: Global Pediatric Vaccines Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: North America Pediatric Vaccines Market Revenue (Million), by Vaccine Type 2025 & 2033
- Figure 3: North America Pediatric Vaccines Market Revenue Share (%), by Vaccine Type 2025 & 2033
- Figure 4: North America Pediatric Vaccines Market Revenue (Million), by Technology 2025 & 2033
- Figure 5: North America Pediatric Vaccines Market Revenue Share (%), by Technology 2025 & 2033
- Figure 6: North America Pediatric Vaccines Market Revenue (Million), by Application 2025 & 2033
- Figure 7: North America Pediatric Vaccines Market Revenue Share (%), by Application 2025 & 2033
- Figure 8: North America Pediatric Vaccines Market Revenue (Million), by Country 2025 & 2033
- Figure 9: North America Pediatric Vaccines Market Revenue Share (%), by Country 2025 & 2033
- Figure 10: Europe Pediatric Vaccines Market Revenue (Million), by Vaccine Type 2025 & 2033
- Figure 11: Europe Pediatric Vaccines Market Revenue Share (%), by Vaccine Type 2025 & 2033
- Figure 12: Europe Pediatric Vaccines Market Revenue (Million), by Technology 2025 & 2033
- Figure 13: Europe Pediatric Vaccines Market Revenue Share (%), by Technology 2025 & 2033
- Figure 14: Europe Pediatric Vaccines Market Revenue (Million), by Application 2025 & 2033
- Figure 15: Europe Pediatric Vaccines Market Revenue Share (%), by Application 2025 & 2033
- Figure 16: Europe Pediatric Vaccines Market Revenue (Million), by Country 2025 & 2033
- Figure 17: Europe Pediatric Vaccines Market Revenue Share (%), by Country 2025 & 2033
- Figure 18: Asia Pacific Pediatric Vaccines Market Revenue (Million), by Vaccine Type 2025 & 2033
- Figure 19: Asia Pacific Pediatric Vaccines Market Revenue Share (%), by Vaccine Type 2025 & 2033
- Figure 20: Asia Pacific Pediatric Vaccines Market Revenue (Million), by Technology 2025 & 2033
- Figure 21: Asia Pacific Pediatric Vaccines Market Revenue Share (%), by Technology 2025 & 2033
- Figure 22: Asia Pacific Pediatric Vaccines Market Revenue (Million), by Application 2025 & 2033
- Figure 23: Asia Pacific Pediatric Vaccines Market Revenue Share (%), by Application 2025 & 2033
- Figure 24: Asia Pacific Pediatric Vaccines Market Revenue (Million), by Country 2025 & 2033
- Figure 25: Asia Pacific Pediatric Vaccines Market Revenue Share (%), by Country 2025 & 2033
- Figure 26: Middle East and Africa Pediatric Vaccines Market Revenue (Million), by Vaccine Type 2025 & 2033
- Figure 27: Middle East and Africa Pediatric Vaccines Market Revenue Share (%), by Vaccine Type 2025 & 2033
- Figure 28: Middle East and Africa Pediatric Vaccines Market Revenue (Million), by Technology 2025 & 2033
- Figure 29: Middle East and Africa Pediatric Vaccines Market Revenue Share (%), by Technology 2025 & 2033
- Figure 30: Middle East and Africa Pediatric Vaccines Market Revenue (Million), by Application 2025 & 2033
- Figure 31: Middle East and Africa Pediatric Vaccines Market Revenue Share (%), by Application 2025 & 2033
- Figure 32: Middle East and Africa Pediatric Vaccines Market Revenue (Million), by Country 2025 & 2033
- Figure 33: Middle East and Africa Pediatric Vaccines Market Revenue Share (%), by Country 2025 & 2033
- Figure 34: South America Pediatric Vaccines Market Revenue (Million), by Vaccine Type 2025 & 2033
- Figure 35: South America Pediatric Vaccines Market Revenue Share (%), by Vaccine Type 2025 & 2033
- Figure 36: South America Pediatric Vaccines Market Revenue (Million), by Technology 2025 & 2033
- Figure 37: South America Pediatric Vaccines Market Revenue Share (%), by Technology 2025 & 2033
- Figure 38: South America Pediatric Vaccines Market Revenue (Million), by Application 2025 & 2033
- Figure 39: South America Pediatric Vaccines Market Revenue Share (%), by Application 2025 & 2033
- Figure 40: South America Pediatric Vaccines Market Revenue (Million), by Country 2025 & 2033
- Figure 41: South America Pediatric Vaccines Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Pediatric Vaccines Market Revenue Million Forecast, by Vaccine Type 2020 & 2033
- Table 2: Global Pediatric Vaccines Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 3: Global Pediatric Vaccines Market Revenue Million Forecast, by Application 2020 & 2033
- Table 4: Global Pediatric Vaccines Market Revenue Million Forecast, by Region 2020 & 2033
- Table 5: Global Pediatric Vaccines Market Revenue Million Forecast, by Vaccine Type 2020 & 2033
- Table 6: Global Pediatric Vaccines Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 7: Global Pediatric Vaccines Market Revenue Million Forecast, by Application 2020 & 2033
- Table 8: Global Pediatric Vaccines Market Revenue Million Forecast, by Country 2020 & 2033
- Table 9: United States Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 10: Canada Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 11: Mexico Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 12: Global Pediatric Vaccines Market Revenue Million Forecast, by Vaccine Type 2020 & 2033
- Table 13: Global Pediatric Vaccines Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 14: Global Pediatric Vaccines Market Revenue Million Forecast, by Application 2020 & 2033
- Table 15: Global Pediatric Vaccines Market Revenue Million Forecast, by Country 2020 & 2033
- Table 16: Germany Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 17: United Kingdom Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 18: France Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 19: Italy Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 20: Spain Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 21: Rest of Europe Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 22: Global Pediatric Vaccines Market Revenue Million Forecast, by Vaccine Type 2020 & 2033
- Table 23: Global Pediatric Vaccines Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 24: Global Pediatric Vaccines Market Revenue Million Forecast, by Application 2020 & 2033
- Table 25: Global Pediatric Vaccines Market Revenue Million Forecast, by Country 2020 & 2033
- Table 26: China Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 27: Japan Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 28: India Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 29: Australia Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 30: South Korea Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 31: Rest of Asia Pacific Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 32: Global Pediatric Vaccines Market Revenue Million Forecast, by Vaccine Type 2020 & 2033
- Table 33: Global Pediatric Vaccines Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 34: Global Pediatric Vaccines Market Revenue Million Forecast, by Application 2020 & 2033
- Table 35: Global Pediatric Vaccines Market Revenue Million Forecast, by Country 2020 & 2033
- Table 36: GCC Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 37: South Africa Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 38: Rest of Middle East and Africa Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 39: Global Pediatric Vaccines Market Revenue Million Forecast, by Vaccine Type 2020 & 2033
- Table 40: Global Pediatric Vaccines Market Revenue Million Forecast, by Technology 2020 & 2033
- Table 41: Global Pediatric Vaccines Market Revenue Million Forecast, by Application 2020 & 2033
- Table 42: Global Pediatric Vaccines Market Revenue Million Forecast, by Country 2020 & 2033
- Table 43: Brazil Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 44: Argentina Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
- Table 45: Rest of South America Pediatric Vaccines Market Revenue (Million) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Pediatric Vaccines Market?
The projected CAGR is approximately 6.34%.
2. Which companies are prominent players in the Pediatric Vaccines Market?
Key companies in the market include Sanofi SA, Sinovac Biotech Ltd, Indian Immunologicals Limited, Mitsubishi Tanabe Pharma Corporation, Merck & Co Inc, Novavax Inc *List Not Exhaustive, Seqirus (CSL Limited), AstraZeneca plc, GlaxoSmithKline PLC, Pfizer Inc.
3. What are the main segments of the Pediatric Vaccines Market?
The market segments include Vaccine Type, Technology, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD 20.29 Million as of 2022.
5. What are some drivers contributing to market growth?
Rising Burden of Chronic Diseases with the Importance and Awareness of Immunization; Increase in the Government and Non-government Funding in R&D.
6. What are the notable trends driving market growth?
The Conjugate Vaccine Segment is Expected to Grow at a Significant Rate.
7. Are there any restraints impacting market growth?
Cost of Immunization; Lesser Medical Coverage and Healthcare Services in Low- and Middle-income Countries.
8. Can you provide examples of recent developments in the market?
In June 2022 Pfizer and BioNTech received the United States Food and Drug Administration received emergency use authorization of the Pfizer-BioNTech COVID-19 vaccine as a three-dose series for children 6 months through 4 years of age.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Pediatric Vaccines Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Pediatric Vaccines Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Pediatric Vaccines Market?
To stay informed about further developments, trends, and reports in the Pediatric Vaccines Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
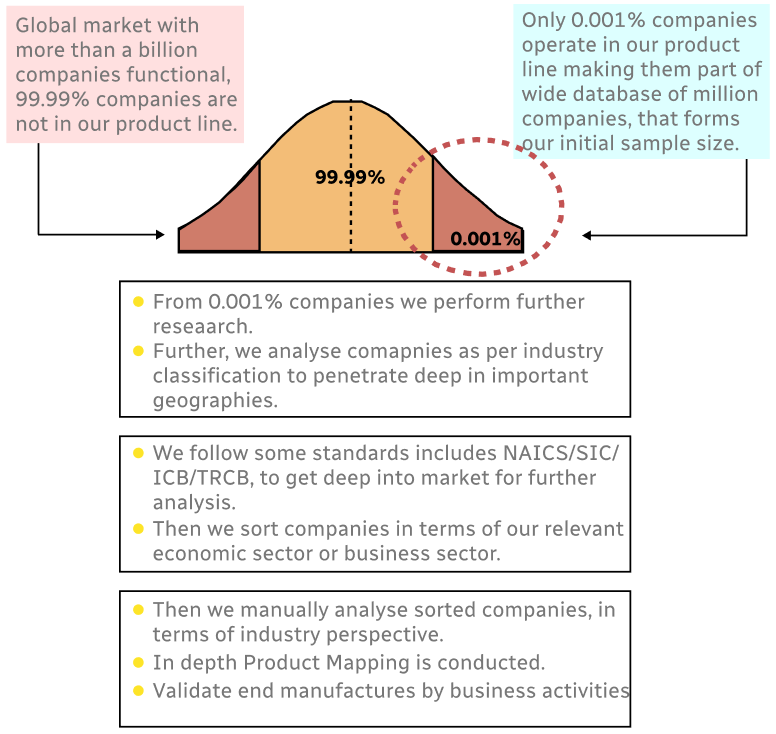
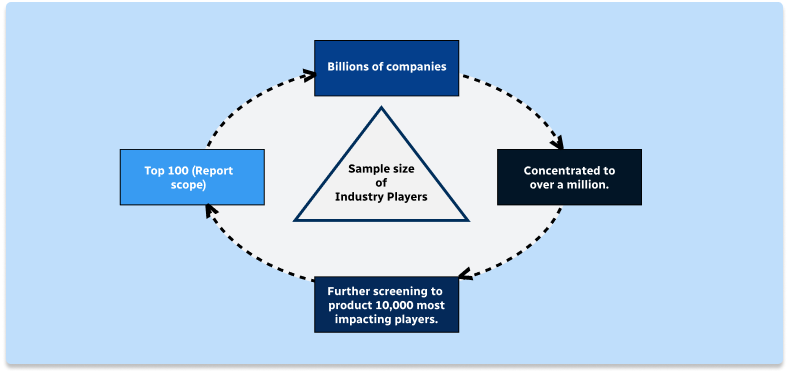
Step 1 - Identification of Relevant Samples Size from Population Database
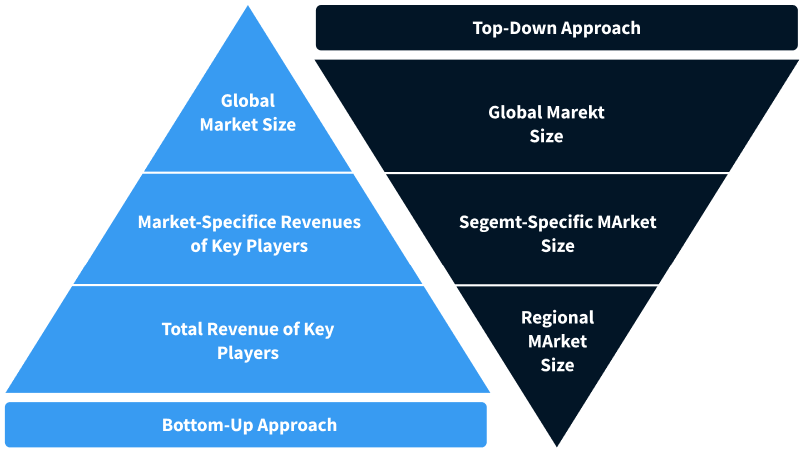
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


